During 2010 there was unprecedented activity in the pricing and market access landscape, much of which has aided the growth in the generics industry during 2010. This activity occurred across three broad themes:
The growth in the generics industry
Home/Reports
|
Posted 08/07/2011
 0
Post your comment
0
Post your comment

Broadening access and affordability
- Widening of healthcare coverage to larger populations
- Increased access to innovative health care
- Access to healthcare infrastructures
- European pharmerging markets: Poland, Romania, Turkey, Ukraine
Increasing complexity
- Diversification of regional environments – decentralisation has led to the appearance of new influential stakeholders
- Divergence of enforcement and implementation of cost containment strategies
New cost containment measures
- Systematic drug evaluation/rationalisation of drug use
- Set up of mechanisms for manufacturers to contribute to system, e.g. risk sharing, collaborations
- Taking of pricing and market access responsibility by new decision makers
- Price cuts
Growth in generics between 2004 and 2008 has consistently outperformed the overall prescription medicines’ market growth. In Canada and the US growth between 2004 and 2009 in generics was 12%, while in the pharmerging market of Japan it was a staggering 14%.
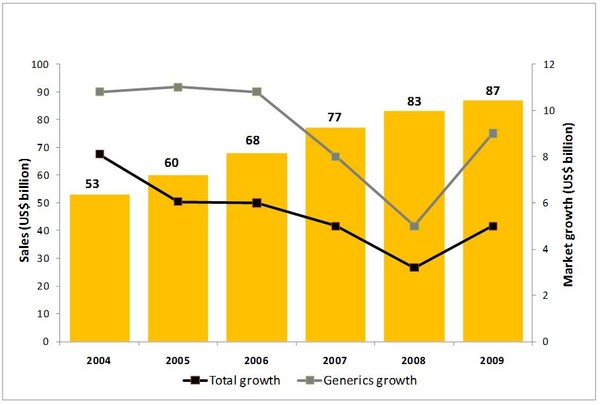
Figure 1: Global pharmaceutical sales 2004-2009
Source: IMS Health, MIDAS, Market Segmentation, MAT Dec 2009.
The global generics market in 2009 reached US$87 billion while growth climbed to 9%, far outstripping that of the total market and also the generic growth in 2008, both of which were around 5%.
The power of payers – such as healthcare funds – has also contributed to this increase in generic uptake, with price and ‘value-for-money’ becoming increasingly important. The power of patients to choose has also been increasing in the past few years, whereas prescribers are seeing a loss of power. Patients are increasingly being empowered to decide whether they will accept or demand generic substitution and more and more governments are demanding that prescribers use generic or INN names when prescribing.
Related articles
Not only generics makers are well placed to move into biosimilars
Diversification of Big Pharma into generics and biosimilars
Patent cliff and the generics industry
Big Pharma and the generics industry
Reference
1. Sheppard A. Generics; opportunities for some, threats for others: strategy shifts and new business models as a consequence. 5th Annual Generics Asia Summit 2010; 2010 Oct 25–26; Singapore.
Source: IMS Health
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment