Global spending on medicines is expected to reach the US$1 trillion threshold in 2014 and rise to US$1.2 trillion by 2017.
The global biologicals market
Home/Reports
|
Posted 13/06/2014
 0
0

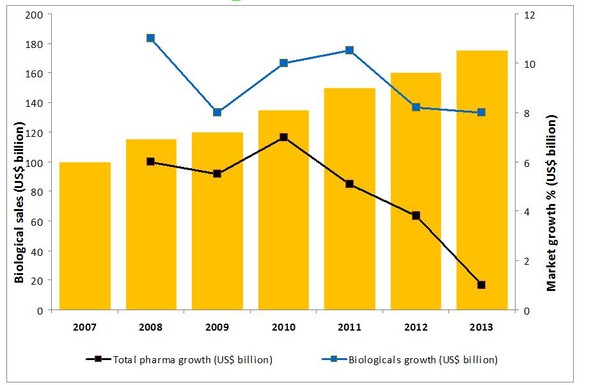
This increase is mainly attributed to greater access to medicines by the world’s rapidly expanding middle class, together with stronger economic prospects in developed nations. However, biologicals may also be attributing to this trend, with spending on biologicals having almost doubled since 2007 and growth outstripping that of total sales of pharmaceuticals by a significant margin, see Figure 1 [1].
Figure 1: Biologicals sales 2007–2013
Source: IMS Health, MIDAS, MAT June 2013
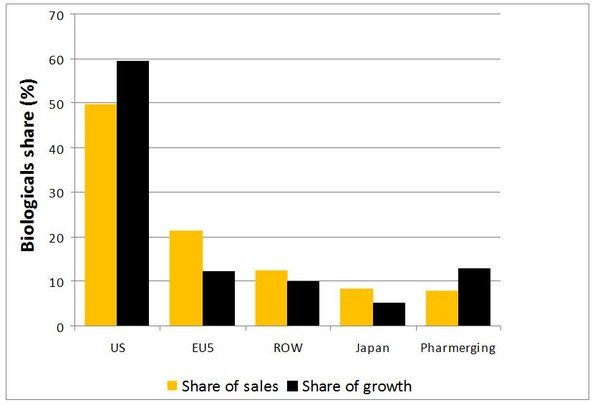
When this is broken down into regions it can be seen that in some regions of the world biologicals make up an even greater percentage of both pharmaceutical sales and growth, see Figure 2.
Figure 2: Biologicals market share
Source: IMS Health, MIDAS, MAT June 2013
EU5: France, Germany, Italy, Spain and UK; ROW: Rest of world; Pharmerging: Argentina, Brazil, China, Egypt, India, Indonesia, Mexico, Pakistan, Poland, Romania, Russia, South Africa, Thailand, Turkey, Ukraine, Venezuela, Vietnam.
The increasing cost of medicines, and especially biologicals, is putting increasing strain on healthcare budgets, but represents an opportunity for lower-cost biosimilars.
Related article
Biologicals dominate Europe’s best sellers
Reference
1. Sheppard A. Biological/biotechnological and biosimilars market: the global outlook with special focus on Europe. 12th EGA International Biosimilar Medicines Conference; 3-4 April 2014; London, UK.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2013 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets