Switching patients from originator biologicals is often an emotive subject. However, despite reservations by prescribers and payers alike the tide may be finally turning, with Scandinavian countries leading the way [1].
The evolution of switching and substitution of biosimilars in Europe
Home/Reports
|
Posted 20/10/2017
 0
Post your comment
0
Post your comment

Switching is defined as a decision by the treating physician to exchange one medicine for another medicine with the same therapeutic intent in patients who are undergoing treatment. Substitution, on the other hand, is the practice of dispensing one medicine instead of another equivalent and interchangeable medicine in any given patient at the pharmacy level without consulting the prescriber.
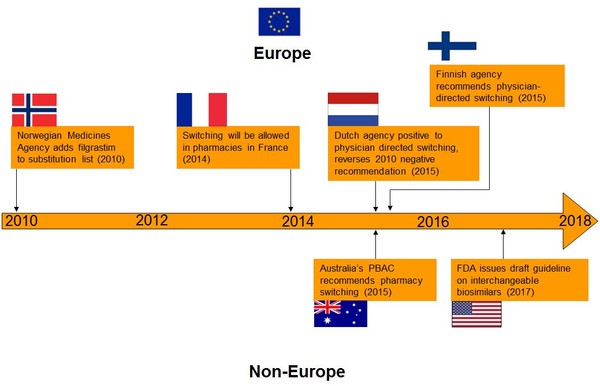
Just a few of the significant decisions surrounding switching and substitution are presented in Figure 1. These include when the Norwegian Medicines Agency added filgrastim to the substitution list in 2010 [2] and when, in 2014, France introduced (as part of a new law concerning the social security budget) biosimilar substitution under certain conditions: at treatment initiation and if not prohibited by the prescriber [3]. In 2015, the Medicines Evaluation Board (MEB) in The Netherlands reversed its 2010 negative recommendation to give a positive recommendation to physician-directed switching [4].
Figure 1: Switching and substitution approaches around the world
FDA: US Food and Drug Administration; PBAC: Pharmaceutical Benefits Advisory Committee.
Other important decisions made recently that affect switching and substitution include the recommendation by the Finnish Medicines Agency, FIMEA, which announced that it considers EU biosimilars interchangeable with their reference biologicals. Automatic substitution at the pharmacy level, however, is not included in the current FIMEA recommendation [5, 6]. Australia’s Pharmaceutical Benefits Advisory Committee (PBAC) also recommended in 2015 that biosimilars are suitable for substitution at the pharmacy level [7]. Then in 2017, the US Food and Drug Administration (FDA) finally issued its much anticipated draft guideline on the interchangeability of biosimilars with their reference biologicals [8].
Related article
Switching to biosimilars in Nordic countries
References
1. Madsen S. Improving access to modern therapies: what can we learn from gainsharing practices? The Scandinavian experience. 15th Biosimilar Medicines Conference. Biosimilar medicines: a game changer for healthcare sustainability; 23−24 March 2017; London, UK.
2. GaBI Online - Generics and Biosimilars Initiative. Norway, biosimilars in different funding systems [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Oct 20]. Available from: www.gabionline.net/Biosimilars/Research/Norway-biosimilars-in-different-funding-systems
3. GaBI Online - Generics and Biosimilars Initiative. France to allow biosimilars substitution [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Oct 20]. Available from: www.gabionline.net/Policies-Legislation/France-to-allow-biosimilars-substitution
4. GaBI Online - Generics and Biosimilars Initiative. Biosimilar substitution in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Oct 20]. Available from: www.gabionline.net/Reports/Biosimilar-substitution-in-Europe
5. GaBI Online – Generics and Biosimilars Initiative. Finnish drug regulator recommends interchangeability of biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Oct 20]. Available from: www.gabionline.net/Policies-Legislation/Finnish-drug-regulator-recommends-interchangeability-of-biosimilars
6. FIMEA. Interchangeability of biosimilars – position of Finnish Medicines Agency Fimea. 22 May 2015 [homepage on the Internet]. [cited 2017 Oct 20]. Available from: www.fimea.fi/documents/542809/838272/29197_Biosimilaarien_vaihtokelpoisuus_EN.pdf
7. GaBI Online – Generics and Biosimilars Initiative. Australia’s PBAC recommends substitution of biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Oct 20]. Available from: www.gabionline.net/Biosimilars/General/Australia-s-PBAC-recommends-substitution-of-biosimilars
8. GaBI Online – Generics and Biosimilars Initiative. FDA issues draft guidance on biosimilar interchangeability [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Oct 20]. Available from: www.gabionline.net/Guidelines/FDA-issues-draft-guidance-on-biosimilar-interchangeability
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2017 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment