The US has come a long way since the introduction of the Biologics Price Competition and Innovation Act of 2009 (BPCI Act), which was signed into law on 23 March 2010 by President Barack Obama.
The evolution of biosimilars in the US
Home/Reports
|
Posted 07/04/2017
 0
Post your comment
0
Post your comment

It has, however, been rather slow on the biosimilars front. In the European Union (EU), a legal framework for approving biosimilars was established in 2003 and biosimilars have been approved by the European Medicines Agency (EMA) since 2006. There are currently 29 biosimilars approved in Europe [1].
In contrast, as of November 2016, the US Food and Drug Administration (FDA) had only approved four biosimilars [2] and had seven active biosimilar filings pending review [3].
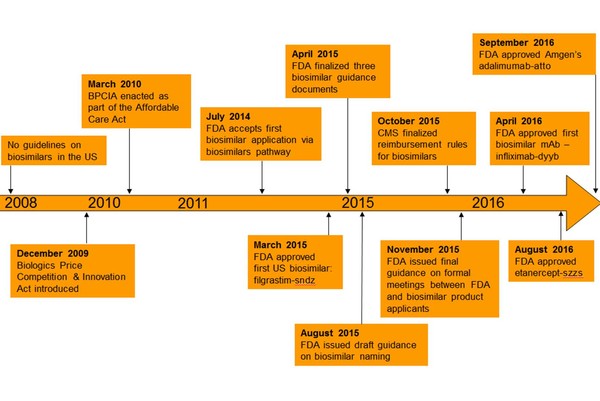
In fact, legislation to allow the approval of biosimilars was only introduced in the US in 2009, see Figure 1.
Figure 1: Evolution of biosimilars in the US
CMS: Centers for Medicare & Medicaid Services; FDA: US Food and Drug Administration.
Despite the introduction of legislation allowing for an abbreviated pathway for biosimilars in the US, FDA only finalized its overarching guidelines on biosimilars in April 2015 [4]. This was after the first biosimilar (filgrastim-sndz) had been approved in the country.
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Apr 7]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Apr 7]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-the-US
3. Jordan B. Regulatory implications for implementing biosimilar interchangeability: addressing policy and practical concerns. SMi 3rd Annual Biosimilars North America Conference; 16−17 November 2016, Iselin, New Jersey, USA.
4. GaBI Online - Generics and Biosimilars Initiative. US guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Apr 7]. Available from: www.gabionline.net/Guidelines/US-guidelines-for-biosimilars
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2017 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment