The 2020 Biosimilars Trend Report opens by stating that the US marketplace is ready to welcome many new biosimilars in 2020 and the years to come [1]. Recent US regulations have levelled the playing field for biosimilars and reference products. For example, the Centers for Medicare and Medicaid Services (CMS) has made changes to the US reimbursement system which has established a Healthcare Common Procedure Coding System (HCPCS) and payment rates for biosimilars. Such changes are hoped to foster competition and create a more sustainable marketplace.
The US biosimilars market in 2020
Home/Reports
|
Posted 27/11/2020
 0
Post your comment
0
Post your comment
An overview of biosimilar trends
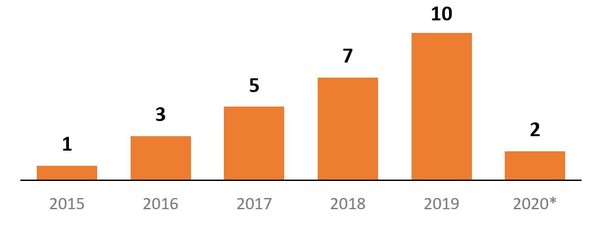
The report highlights that, in 2018, the US Food and Drug Administration (FDA) approved seven biosimilars, and in 2019 it approved 10, bringing the total approved to 26. Due to the COVID-19 pandemic, a slowdown of biosimilar approvals is expected in 2020, but two were approved prior to July 2020. As of July 2020, 28 biosimilars were approved, see Figure 1.
Figure 1: Number of approved biosimilars in the US, per year
*2020 totals only include January to July.
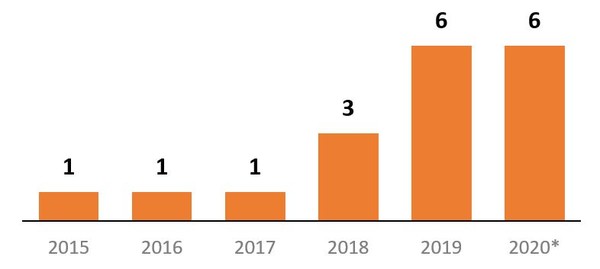
When a biosimilar is approved in the US it does not immediately become available to patients, and between 2015‒2017, only one biosimilar became available each year. However, in 2019, and up to July 2020, six biosimilars have become available to patients each year. As of July 2020, 18 biosimilars were available, see Figure 2.
Figure 2: Number of biosimilars becoming available in the US, per year
*2020 totals only include January to July.
The approved biosimilars are for nine reference products, these are filgrastim, pegfilgrastim, bevacizumab, trastuzumab, infliximab, epoetin alfa, rituximab, etanercept and adalimumab. Seven of these were available when the report was compiled.
The report highlights that, over the last year, the number of biosimilar approvals has increased by 65% and the number of available biosimilars has increased by 157%.
Comparison to the EU
The report also draws comparisons to the European Union (EU) where the first biosimilar was approved in 2006. In the five years following this, 11 biosimilars were approved in the EU. In the US, 26 biosimilars were approved in the five years after the first US approval in 2015.
The EU has seen widespread adoption of biosimilars in the last 13 years which has led to consistent price reduction. It is expected that biosimilar competition in the US will also lead to this and savings can be reinvested.
Biosimilar prices
The report notes that now, some manufactures are launching biosimilars at wholesale acquisition costs (WACs), that are up to 37% lower than those of the reference product. Almost all biosimilars are being launched at a WAC price between 3%‒24% less than the average sales price (ASP) of the reference product.
Competition from biosimilars is starting to show that the ASP of reference products is declining. ASPs for biosimilars are also declining. For example, the ASP for the infliximab reference product (Remicade) has reduced by over 30% and the ASPs for the biosimilars, Inflectra and Renflexis, have reduced by approximately 40%.
The rate of biosimilar uptake is increasing over time. They now have a significant share in the therapeutic areas to which they have been introduced. The share of Neupogen (filgrastim) biosimilars is almost 75% after 5 years.
Related articles
Approval and launch dates for US biosimilars
Barriers to biosimilars access in the US
FDA releases new information on interchangeable biologicals
Reference
1. Amgen Biosimilars. 2020 Biosimilars trends report [homepage on the Internet]. [citied 2020 Nov 27]. Available from: https://www.amgenbiosimilars.com/-/media/Themes/Amgen/amgenbiosimilars-com/Amgenbiosimilars-com/pdf/USA-CBU-80723-2020-Amgen-Biosimilar-Trends-Report.pdf
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment