Measures for promoting the rational use of medicines in the 27 EU Member States were surveyed in a report by the Gesundheit Österreich GmbH / Österreichisches Bundesinstitut für Gesundheitswesen (GÖG/ÖBIG – Austrian Health Institute).
Survey on rational use of medicines in 27 EU Members States
Home/Reports
|
Posted 22/04/2011
 0
Post your comment
0
Post your comment
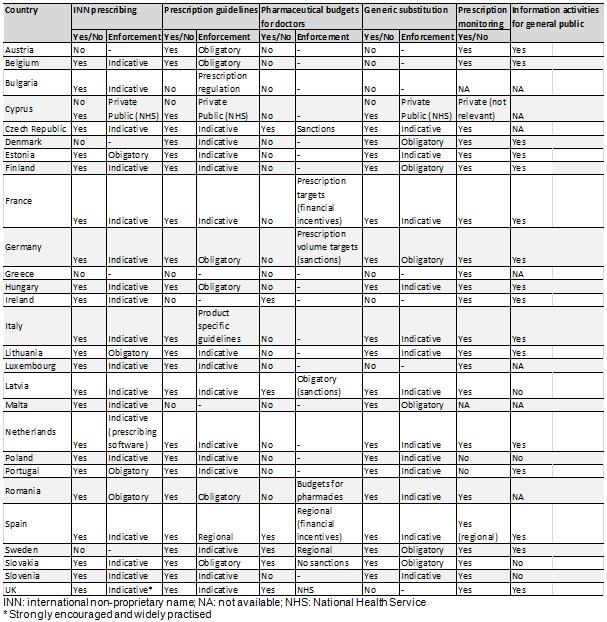
Their results showed that there are many different practices between the different Member States in the EU with regard to generic medicine policies. An overview is given in the table below:
For example, while most countries (22) advocate international non-proprietary name (INN) prescribing, relatively few (4) have made this obligatory, whereas generic substitution by the pharmacist is practiced in the majority of countries (21).
Acknowledgement
This article is published with permission of the Gesundheit Österreich GmbH (GÖG)/Austrian Health Institute.
We gratefully acknowledge the support from Dr Sabine Vogler of Gesundheit Österreich GmbH / Österreichisches Bundesinstitut für Gesundheitswesen (GÖG/ÖBIG – Austrian Health Institute) to GaBI Online.
Related articles
The EU and rational use of medicines
The Netherlands and rational use of medicines
Italy’s rational use of medicines
France’s rational use of medicines
Germany’s rational use of medicines
Rational use of medicines in Denmark
Promoting rational use of medicines in Europe
Reference
Vogler S, Schmickl B. Rational use of medicines in Europe. Gesundheit Österreich GmbH / Österreichisches Bundesinstitut für Gesundheitswesen (GÖG/ÖBIG). February 2010.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment