In an information guide for healthcare professionals jointly prepared by the European Medicines Agency (EMA) and the European Commission (EC), interchangeability refers to the possibility of exchanging one medicine for another medicine that is expected to have the same clinical effect. In terms of biologicals, this could mean replacing a reference product with a biosimilar (or vice versa) or replacing one biosimilar with another [1].
Interchangeability and switching study designs for biosimilars
Home/Reports
|
Posted 05/01/2018
 1
Post your comment
1
Post your comment

Also, interchangeability can be defined as ‘the medical practice of changing one medicine for another that is expected to achieve the same clinical effect in a given clinical setting and in any patient on the initiative, or with the agreement of the prescriber’ [2].
How to establish interchangeability and selection of possible designs of switching studies for biosimilars were discussed by Dr Hans Ebbers of the Medicines Evaluation Board (MEB/CBG) in The Netherlands at the European stakeholder event on biosimilars in Brussels, Belgium [3].
One important factor to consider when it comes to interchangeability is whether switching between originator biologicals and biosimilars or between different biosimilars can increase the risk of anti-drug antibodies, which can lead to immunological reactions (type-I hypersensitivity and injection site or infusion reactions) and decreased drug efficacy (loss of response).
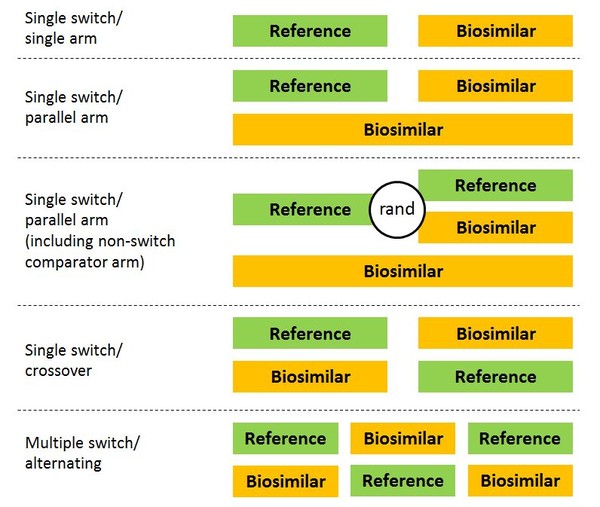
But what should studies investigating switching to biosimilars look like? Several designs have been suggested, see Figure 1.
Figure 1: Possible designs for switching studies of biosimilars
rand: randomized.
The problem with single-arm studies, however, is that they are often hard to interpret, and even well-designed trials may not be sensitive enough to detect small differences in efficacy. However, despite the limitations of switching studies, a review of switching data for biosimilars ‘found no evidence from clinical trial data or post marketing surveillance data that switching to and from different biopharmaceuticals leads to safety concerns’ [4].
And Pekka et al. concluded that ‘a state-of-the-art demonstration of biosimilarity, together with intensified post-marketing surveillance, is a sufficient and realistic way of ensuring interchangeability of EU-approved biosimilars under supervision of the prescriber’ [5].
Disclaimer
Dr Hans Ebbers stated that the views expressed in his presentation are the personal views of the presenter and are not to be understood or quoted as being made on behalf of, or reflecting the position of the MEB/CBG or any other regulatory agency, or one of its committees or working parties.
Related articles
Establishing interchangeability for biosimilars in the US
Establishing interchangeability for biosimilars
References
1. Biosimilars in the EU - Information guide for healthcare professionals. Available from: http://ec.europa.eu/DocsRoom/documents/22924
2. GaBI Online – Generics and Biosimilars Initiative. Integrating biosimilars into clinical practice [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Jan 5]. Available from: www.gabionline.net/Biosimilars/Research/Integrating-biosimilars-into-clinical-practice
3. European Commission Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs. Multi-stakeholder Workshop on Biosimilar Medicinal Products; 5 May 2017; Brussels, Belgium.
4. Ebbers et al. The safety of switching between therapeutic proteins. Expert Opin Biol Ther 2012;12(11):1473-85.
5. Kurki et al. Interchangeability of Biosimilars: A European Perspective. BioDrugs. 2017;31(2):83-91.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2018 Pro Pharma Communications International. All Rights Reserved.
Posted 08/01/2018 by Paul Declerck
Interchangeability does not refer to a medical practice but to a property of two drugs
First posted 8/1/2018 ---
Very nice presentation of Dr Ebbers with very useful information.
It may be needed to stress that in the discussion on interchangeability/switching/substitution the EMA as well as many authors provide an erroneous definition of interchangeability. Indeed, whereas ‘switching’ and ‘substitution’ refer to a “practice” (of the physician and the pharmacist, respectively) ‘interchangeability’ does not refer to a medical practice but actually refers to a property of two drugs. Two drugs are interchangeable if changing one drug for the other is expected to achieve the same clinical effect in a given clinical setting and in any patient. And, important in the context of biosimilars, one should also take into account that the designation of ‘interchangeable’ can only be attributed if also alternating (i.e., back-and-forth!) between the two drugs does not affect either the safety or the efficacy in any patient. This is also the reason why the approach of the FDA with respect to the requirements for the designation of ‘interchangeable’ is scientifically correct [1]. ---
[1] Declerck Paul, Endrenyi Laszlo, Chow Shein-Chung (2017). Interchangeability, Switchability, and Substitution of Biosimilar Products. In: Endrenyi Laszlo, Declerck Paul, Chow Shein-Chung (Eds.),
Biosimilar Drug Product Development, (Chapter 10, pp 283-296) CRC Press.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment