The number of players in the biosimilars field is increasing with both originator and generics manufacturers devoting resources to biosimilars. This applies to global companies as well as to national players [1].
Global and national biosimilars players increasing
Home/Reports
|
Posted 03/10/2014
 3
Post your comment
3
Post your comment
The high growth rates in the global biologicals market and the huge gains to be made from making biosimilars of these blockbusters is fuelling growth, but many challenges remain. Despite this, the number of companies entering the biosimilars arena is expanding.
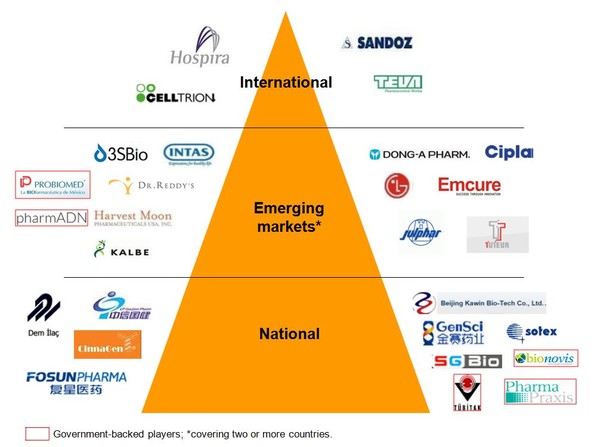
Just a few of the many players now busy with biosimilars can be seen in Figure 1.
The companies entering the biosimilars field differ not only in their geographical spread but also in their wider biologicals portfolio. This is not surprising when considering the possibility of huge rewards. According to research firm Datamonitor, US$60 billion in annual sales will lose patent protection by 2015 [2].
It should be noted that biosimilars approved in countries outside the EU may not have been authorized following as strict a regulatory process as is required for biosimilars in the EU. The EMA (European Medicines Agency) regulatory requirements ensure the same high standards of quality, safety and efficacy for biosimilars as for originator biologicals, and also include a rigorous comparability exercise with the reference product.
Editor’s Comment
If you would like to receive a high resolution copy of Figure 1* please send us an email.
*For profit organizations subjected to a fee
Related articles
Biosimilars in emerging markets
Biosimilars approved in Europe
Not only generics makers are well placed to move into biosimilars
References
1. Sheppard A. Biological/biotechnological and biosimilars market: the global outlook with special focus on Europe. 12th EGA International Symposium on Biosimilar Medicines; 3-4 April 2014; London, UK.
2. GaBI Online - Generics and Biosimilars Initiative. Generics manufacturers and biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Oct 3]. Available from: www.gabionline.net/Biosimilars/News/Generics-manufacturers-and-biosimilars
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2014 Pro PharmaCommunications International. All Rights Reserved.
Posted 02/06/2015 by Bioconsultant
Figure with national/international and emerging markets
which countries do you mean when you list national and international. Thanks
Posted 14/11/2014 by Justyna K, GaBI Online Editorial Office
Response to ‘Domestic’
Dear John, Thank you for your comment/. We updated the word ' domestic ' to national for clearer understanding. Kind regards, Justyna
Posted 08/10/2014 by John
Domestic
By domestic, which country is meant?
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment