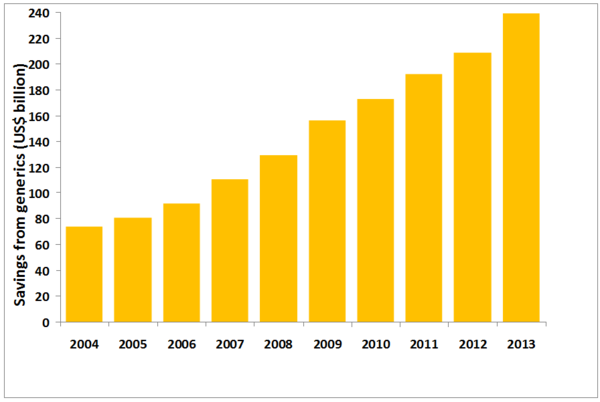
Generics have saved consumers and the US healthcare system US$239 billion in 2013 and have saved the US nearly US$1.5 trillion between 2004 and 2013, according to a report by the Generic Pharmaceutical Association (GPhA) [1].
Generics save US 1.5 trillion over last 10 years
Home/Reports
|
Posted 24/10/2014
 0
Post your comment
0
Post your comment

Spending on health care in the US has slowed down. Medicare spending is projected to drop by $49 billion (less than 1%) and Medicaid spending is expected to drop by $40 billion (approximately 1%) from 2015 to 2024. The GPhA believes that generics have played a key role in the downturn of rising health costs.
The savings in 2013 represent a 14% increase over cost savings achieved in 2012, and the largest annual savings to date, showing that generics use is vital to holding down health costs, see Table 1.
Table 1: Healthcare savings due to generics use in the US (2004–2013)
Source: GPhA
In 2013, savings from newer generics – defined as those entering the market in the past 10 years – accounted for US$140 billion (59%) of total savings. This compares to US$157 billion (72%) of the total US$217 billion of generics savings made in 2012. The majority of the cost savings come from nervous system and cardiovascular treatments, which accounted for 57% of all savings in 2013, and 58% or US$851 billion of cost savings over the last 10 years.
Savings from newer generics are expected to continue to grow over the coming years as patents expire on currently protected brand-name drugs. Between 2014 and 2016, brand-name drugs with US$40 billion in annual sales will be exposed to competition from generics in the US.
Related article
Generics reach 80% and play a critical role in reducing costs
Reference
1. Generic Pharmaceutical Association (GPhA). Generic Drug Savings in the U.S. Sixth Annual Edition: 2014.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2014 Pro PharmaCommunications International. All Rights Reserved.
Source: GPhA
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment