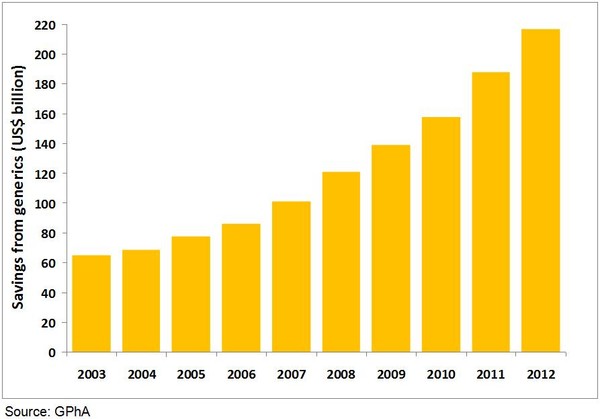
Generics have saved consumers and the US healthcare system US$217 billion in 2012 and have saved the US in excess of US$1.2 trillion between 2003 and 2012, according to a report by the Generic Pharmaceutical Association (GPhA) [1].
Generics save US 1.2 trillion over last 10 years
Home/Reports
|
Posted 14/02/2014
 0
Post your comment
0
Post your comment

Spending on health care in the US in 2011 grew by 3.9% to reach US$2.7 trillion or US$8,680 per person. The rate of growth in healthcare spending in 2011 was the same as in 2009 and 2010. Spending on health care represents nearly 18% of the US Gross Domestic Product (GDP).
The annual rate of increase in savings generated by generics in the US has averaged approximately 17% since 2007, showing that generics use is vital to holding down health costs, see Table 1.
Table 1: Healthcare savings due to generics use in the US (2003–2012)
In 2012, savings from newer generics – defined as those entering the market in the past 10 years – accounted for US$157 billion (72%) of total savings. This compares to US$123 billion (65%) of the total US$188 billion of generics savings made in 2011. The majority of the cost savings come from nervous system and cardiovascular treatments, which accounted for 60% of all savings in 2012.
The report shows that savings to Medicare Part D plans reached approximately US$180 billion since the Part D program began in 2006, while Medicaid savings totalled more than US$96 billion. During this same period, generics have saved out-of-pocket cash payers US$78 billion, making a critical difference to these typically uninsured and poorer customers.
The reports authors expect savings from newer generics to continue to grow over the coming years as patents expire on currently protected brand-name drugs. Between 2014 and 2016, brand-name drugs with US$40 billion in annual sales will be exposed to competition from generics in the US.
Related article
Generics reach 80% and play a critical role in reducing costs
Reference
1. Generic Pharmaceutical Association (GPhA). Generic Drug Savings in the U.S. Fifth Annual Edition: 2013.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2014 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment