In recent years generics makers are becoming bolder and challenging more patents on originator drugs. In fact, litigation regarding patents on pharmaceuticals is on the rise, particularly when it comes to newer drugs.
Generics makers challenging more patents
Home/Reports
|
Posted 05/12/2014
 0
Post your comment
0
Post your comment

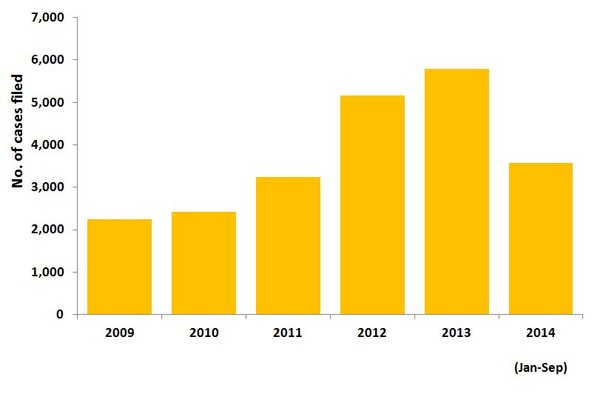
According to a report from legal analysis firm Lex Machina, patent challenges have been rising since 2009. The report finds that 2013 was a record year for abbreviated new drug application (ANDA) law suits and 2014 looks like it is following suit, see Figure 1. In 2013, a total of 5,793 patent cases were filed in the US, compared to a total of 3,564 patent cases filed between January and September 2014.
Figure 1: ANDA patent cases filed per year
ANDA: abbreviated new drug application.
Purdue Pharma’s pain relief medication Oxycontin (oxycodone) has had the largest number of ANDA litigation cases. While testosterone and metformin hydrochloride were the leading ingredients when it came to patent enforcement.
The down side for originator manufacturers is that it also takes longer for a company to gain approval for their drug after their patent has been filed, rising from 3.9 years in 2009 to 4.9 years in 2014.
The report also found that originator companies were likely to face patent attacks on their products from generics much earlier than previously. Currently, the drug patents facing litigation are still fairly new – only five years old on average – compared to an average of 10 years in 2010.
Related article
MSF launches online ‘Patent Opposition Database’
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2014 Pro PharmaCommunications International. All Rights Reserved.
Source: Lex Machina
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment