At the 17th Biosimilar Medicines Conference in The Netherlands, Dr Gillian Woolett of US healthcare consultancy Avalere Health discussed challenges facing the biosimilars market in the US and upcoming changes in the Food and Drug Administration (FDA).
Challenges facing the US biosimilars market
Home/Reports
|
Posted 19/07/2019
 0
Post your comment
0
Post your comment

Although the US has one of the largest biosimilars markets in the world, with the potential to save over US$50 billion on spending on biologicals by 2026, the market continues to face challenges, with only 10 biosimilars launched to date.
| Figure 1: Biosimilars approved in the US | |||||
| Sponsor
|
Brand Name (Proper Name) | Date of Approval | Sponsor
|
Product (Active Ingredient | Date of Approval |
| Sandoz* | Zarxio (filgrastim-sndz) | 6 Mar 2015 | Amgen | Neupogen (filgrastim) | 20 Feb 1991 |
| Celltrion* | Inflectra (infliximab-dyyb) | 5 Apr 2016 | Janssen | Remicade (infliximab) | 24 Aug 1998 |
| Sandoz | Erelzi (etanercept-szzs) | 30 Aug 2016 | Amgen | Enbrel (entanercept) | 2 Nov 1998 |
| Amgen | Amjevita (adalimumab-atto) | 23 Sep 2016 | AbbVie | Humira (Adalimumab) | 31 Dec 2002 |
| Samsung Bioepis* | Renflexis (infliximab-abda) US; (Flixabi®EU) | 21 Apr 2017 | Centocor | Remicade (infliximab) | 24 Aug 1998 |
| Mylan/Biocon | Ogivri (trastuzumab-dkst) | 1 Dec 2017 | Genentech | Herceptin (trastuzumab) | 25 Sep 1998 |
| Amgen/Allergan* | Mvasi (bevacizumab-awwb) | 14 Sep 2017 | Genentech | Avastin (bevacizumab) | 26 Feb 2004 |
| Boehringer Ingelheim | Cyltezo (adalizumab-adbm) | 25 Aug 2017 | AbbVie | Humira (Adalimumab) | 31 Dec 2002 |
| Pfizer | Ixifi (infliximab-qbtx) | 13 Dec 2017 | Centocor | Remicade (infliximab) | 24 Aug 1998 |
| Hospira/Pfizer* | Retacrit (epoetin alfa-epbx) | 15 May 2018 | Amgen | Epogen/Procrit (epoetin alfa) | 1 Jun 1989 |
| Mylan GmbH* | Fulphila (pegfilgrastim-jmdb) | 4 Jun 2018 | Amgen | Neulasta (pegfilgrastim) | 31 Jan 2002 |
| Hospira/Pfizer* | Nivestym (filgrastim-aafi) | 20 Jul 2018 | Amgen | Neupogen (filgrastim) | 20 Feb 1991 |
| Sandoz | Hyrimoz (adalimumab-adaz) | 30 Oct 2018 | AbbVie | Humira (Adalimumab) | 31 Dec 2002 |
| Coherus BioSciences* | Udenyca (pegfilgrastim-cbqv) | 2 Nov 2018 | Amgen | Neulasta (pegfilgrastim) | 31 Jan 2002 |
| Celltrion* | Truxima (rituximab-abbs) | 28 Nov 2018 | Genentech | Rituxan (rituximab) | 26 Nov 1997 |
| Celltrion* | Herzuma (trastuzumab-pkrb) | 14 Dec 2018 | Genentech | Herceptin (trastuzumab) | 25 Sep 1998 |
| Samsung Bioepis | Ontruzant (trastuzumab-dttb) | 18 Jan 2019 | Genentech | Herceptin (trastuzumab) | 25 Sep 1998 |
| Pfizer | Trazimera (trastuzumab-qyyp) | 11 Mar 2019 | Genentech | Herceptin (trastuzumab) | 25 Sep 1998 |
| *Launched. | |||||
A recent presentation by Avalere Health, a consultancy which researches healthcare reform in the US, began by discussing the value of biological drugs to the US public. The US has one of the biggest markets for biological drugs in the world, which is increasing year on year. The total spend for biologicals in the top 20 increased from US$7.7 million in 2012 to US$13.9 million in 2016 (over 90% of total spending for the top 20 drugs, part B).
This is arguably because biological drugs are too expensive in the US. At low prices, biological drugs are uneconomic to produce, but at high prices access to patient populations is reduced. There is thus a need for more sustainable competition amongst biological drugs, regardless of the regulatory pathway by which they were approved.
The presentation by Dr Woolett also discussed recent changes at FDA, including the departure of Commissioner Dr Scott Gottlieb at the end of March 2019. Since Dr Gottlieb joined FDA in 2017, he initiated many changes, including an increase in the number of guidances issued; more product-specific guidance; higher visibility and clinical trials transparency; accelerated initiatives on complex generics; and consideration for broader issues, such as the international context, economics and access. The current acting FDA Commissioner is Dr Norman Sharpless of the National Cancer Institute – where he takes FDA remains to be seen.
Even after FDA approval, there are further challenges for biosimilars, including exclusivity (patenting) and commercialisation. To address these issues, the Trump Administration passed the ‘Biosimilars Action Plan’ in July 2018 to encourage ‘innovation and competition’ among biologicals and the development of biosimilars. In May 2019, FDA released industry guidance on the ‘Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment and Other Quality-Related Considerations’.
Dr Leah Christl, previous Director of the Therapeutic Biologics and Biosimilars Staff in the Office of New Drugs, also released three guidance documents relating to biosimilars. These covered approaches to evaluating similarity, considerations in demonstrating interchangeability, and post-approval manufacturing considerations.
Despite these advances, there should be more focus on the consistent regulation of biologicals in the US, which has been found to sell drugs at many times their cost in Europe. AbbVie’s Humira (Adalimumab) for example, sold for five times its cost in Europe [1], leading some to accuse the company of exploiting patent laws to prevent biosimilars from entering the market.
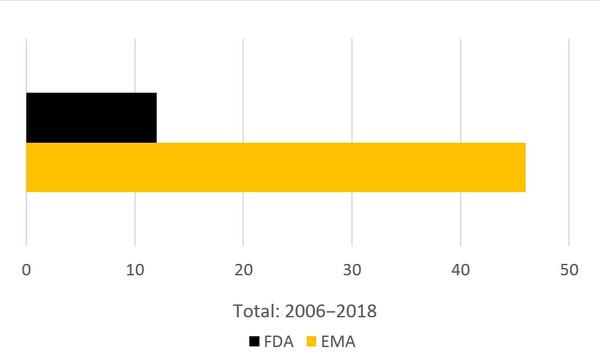
And although the US and Europe have very similar regulatory processes, FDA still lies behind the European Medicines Agency (EMA) in terms of biosimilars approvals, by more than a factor of 4.
Figure 2: Rates of biosimilars approvals in Europe and the US show the US has fewer approvals
Source: EMA: European Medicines Agency, FDA: US Food and Drug Administration, as of 15 June 2019
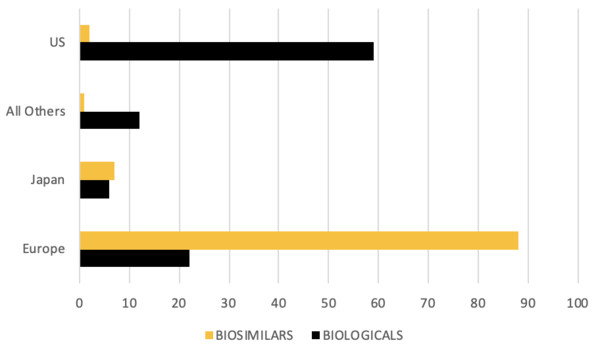
According to Dr Woolett, part of the problem is the variable requirements for data in different regions of the US. However, she also said that biosimilars face challenges globally, as sales worldwide are less than 1% of originator biologicals. There is therefore scope for cost savings not only in the US but worldwide.
Figure 3: Global sales by % total market for biologicals and biosimilars
Related articles
Achieving consistent regulation for biosimilars
FDA issues guidance on quality-related considerations for biosimilars
FDA issues final guidance on interchangeable biologicals
Reference
1. GaBI Online - Generics and Biosimilars Initiative. AbbVie makes more deals delaying adalimumab biosimilars in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Jul 19]. Available from: www.gabionline.net/Pharma-News/AbbVie-makes-more-deals-delaying-adalimumab-biosimilars-in-the-US
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2019 Pro Pharma Communications International. All Rights Reserved.
Source: EMA: European Medicines Agency, FDA: US Food and Drug Administration, as of 15 June 2019
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment