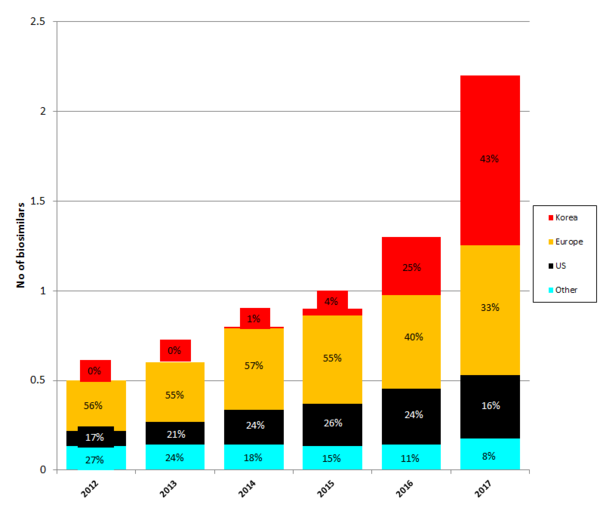
Biosimilars manufacturing is moving to Asia with biosimilars developed in Korea contributing 43% of the global biosimilars value. This has been a quick expansion from the total share of 0% back in 2012. As outlined in Mr Per Troein’s presentation [1] on how the landscape of biosimilars development is changing, Korea has now taken over from Europe as the leading location for biosimilars manufacturing, see Figure 1.
Biosimilars development moving to Asia
Home/Reports
|
Posted 29/06/2018
 0
Post your comment
0
Post your comment

Figure 1: Global biosimilars value originator by region
Source: IQVIA MIDAS Q1 2017, Pipeline: July 2017
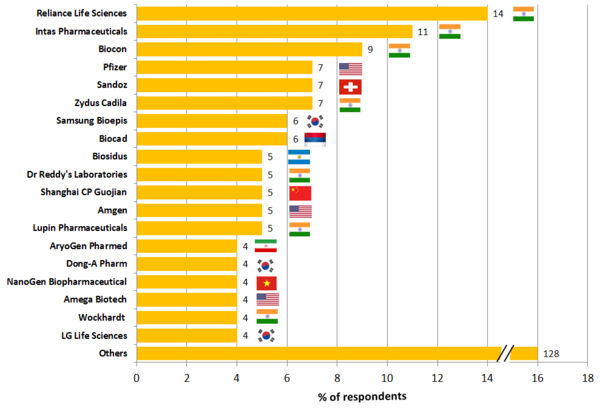
Asia, and in particular India, has long been a player in the generics market. Therefore, it should perhaps not come as a surprise that Indian players have some of the biggest and most advanced pipelines for biosimilars, see Figure 2.
Figure 2: Global biosimilar pipeline by manufacturer (phase III to approved)
Source: IQVIA MIDAS Q1 2017, Pipeline: July 2017
Just some examples of recent biosimilar approvals from South Korean companies include the approval of adalimumab biosimilar Hadlima (SB5) and trastuzumab biosimilar Samfenet (SB3) from Samsung Bioepis in September and November 2017, respectively [2]. In March 2018, South Korea-based GC Pharma (formerly Green Cross) received approval for its insulin glargine biosimilar Glarzia [3]. Then, in the same month, LG Chem (part of LG Life Sciences) also received approval for its etanercept biosimilar, Eucept [4].
India has, by far, demonstrated the greatest acceptance of ‘similar biologics’. According to our research at GaBI Online, the first ‘similar biologic’ was approved and marketed in India for a hepatitis B vaccine in 2000. In recent years over 50 biopharmaceutical products have been approved for marketing in India, with more than half of them being ‘similar biologics’ [5].
Conflict of interest
The author of the presentation [1] did not provide any conflict of interest statement.
Related article
Developing biosimilars
References
1. Troein P. Trends in the development of the biosimilar market. 16th European Medicines Conference – Biosimilar Medicines: unlocking the full potential of biologics; 26-27 April 2018; London, UK.
2. GaBI Online - Generics and Biosimilars Initiative. Adalimumab and trastuzumab biosimilars approved in South Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Jun 29]. Available from: www.gabionline.net/Biosimilars/News/Adalimumab-and-trastuzumab-biosimilars-approved-in-South-Korea
3. GaBI Online - Generics and Biosimilars Initiative. Insulin glargine biosimilar gains South Korean approval [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Jun 29]. Available from: www.gabionline.net/Biosimilars/News/Insulin-glargine-biosimilar-gains-South-Korean-approval
4. GaBI Online - Generics and Biosimilars Initiative. Biosimilar etanercept Eucept approved in South Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Jun 29]. Available from: www.gabionline.net/Biosimilars/News/Biosimilar-etanercept-Eucept-approved-in-South-Korea
5. GaBI Online - Generics and Biosimilars Initiative. ‘Similar biologics’ approved and marketed in India [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Jun 29]. Available from: www.gabionline.net/Biosimilars/General/Similar-biologics-approved-and-marketed-in-India
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2018 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment