In the European Union (EU), decisions on the interchangeability or substitution of biosimilars and originator biologicals are not made by the European Medicines Agency (EMA), but at the national level. This is despite the fact that biosimilars developed in line with EU requirements are considered by EMA to be therapeutic alternatives to their reference biologicals.
Biosimilar substitution in Europe
Home/Reports
|
Posted 26/05/2017
 0
Post your comment
0
Post your comment

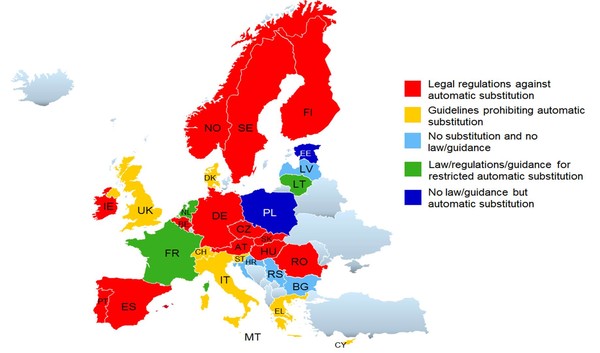
Automatic substitution of biosimilars is therefore not routinely practised. This, according to Dr Niraj Chhaya, Risk Management, Global Pharmacovigilance, Boehringer Ingelheim, is due to concerns regarding traceability and that repeated switching between the biosimilar and reference biological may increase immunogenicity [1]. Although most Member States do not allow automatic substitution, and many have introduced rules to avoid automatic substitution of biosimilars [2], some have allowed limited substitution of biosimilars, see Figure 1.
Figure 1: Biosimilar substitution rules in Europe
In 2013, the Italian Medicines Agency (Agenzia Italiana del Farmaco:AIFA) stated that physicians should consider biosimilars as the preferred option for naïve patients where it positively impacts the healthcare budget. In the same year, France had introduced as part of a new law concerning the social security budget (pending for adoption of the decree) for biosimilar substitution under certain conditions: at treatment initiation and if not prohibited by the prescriber [3]. Greece’s medicines agency [National Organization for Medicines (EOF)], on the other hand, released a document in 2013 recommending against automatic substitution/interchangeability of reference biologicals and their biosimilars [4].
In 2014, the Norwegian Government set up a clinical study to assess the interchangeability of Remicade (infliximab) with its biosimilar – the NOR-SWITCH study. Results of this two year-long phase IV study presented in October 2016, showed that Celltrion’s infliximab biosimilar (Remsima, CT‑P13) is not inferior to the originator biological Remicade [5].
According to the reported results, this two year-long phase IV study presented in October 2016 showed that Celltrion’s infliximab biosimilar (Remsima, CT‑P13) is not inferior to the originator biological Remicade [5].
In 2015, the Finnish Medicines Agency, FIMEA, announced that it considers EU biosimilars interchangeable with their reference biologicals. Automatic substitution at the pharmacy level, however, is not included in the current FIMEA recommendation [6, 7]. Other EU national authorities, including the Medicines Evaluation Board (MEB) in The Netherlands, the Paul-Ehrlich-Institut (PEI) in Germany and the Health Products Regulatory Authority (HPRA) in Ireland [8], have adopted similar positions. In the same year, Portugal published interchangeability regulations that contain an exemption that switching has to be justified based on scientific opinion.
Dr Chhaya concludes that guidelines and rules are being developed ‘slowly and steadily’, adding that ‘in countries without specific provisions or guidelines automatic substitution is being carried out for economic reasons’. Finally, according to Dr Chhaya, the choice of treatment should remain a clinical decision and be entrusted to the prescribing physician.
If you would like to receive a high-resolution copy* of the Figure 1, please send us an email.
*For profit organizations are subjected to a fee
Related article
Interchangeability of biosimilars around the world
References
1. Chhaya N. Robust pharmacovigilance: a key to successful biosimilars. SMi Group. Biosimilars Europe; 29−30 September 2016; London, United Kingdom.
2. GaBI Online - Generics and Biosimilars Initiative. Efficacy, extrapolation and interchangeability of biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 May 26]. Available from: www.gabionline.net/Biosimilars/Research/Efficacy-extrapolation-and-interchangeability-of-biosimilars
3. GaBI Online - Generics and Biosimilars Initiative. France to allow biosimilars substitution [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 May 26]. Available from: www.gabionline.net/Policies-Legislation/France-to-allow-biosimilars-substitution
4. GaBI Online - Generics and Biosimilars Initiative. Greece says no to automatic substitution of biologicals [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 May 26]. Available from: www.gabionline.net/Biosimilars/News/Greece-says-no-to-automatic-substitution-of-biologicals
5. GaBI Online - Generics and Biosimilars Initiative. NOR-SWITCH study finds biosimilar infliximab not inferior to originator [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 May 26]. Available from: www.gabionline.net/Biosimilars/Research/NOR-SWITCH-study-finds-biosimilar-infliximab-not-inferior-to-originator
6. GaBI Online – Generics and Biosimilars Initiative. Finnish drug regulator recommends interchangeability of biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 May 26]. Available from: www.gabionline.net/Policies-Legislation/Finnish-drug-regulator-recommends-interchangeability-of-biosimilars
7. FIMEA. Interchangeability of biosimilars – position of Finnish Medicines Agency Fimea. 22 May 2015 [homepage on the Internet]. [cited 2017 May 26]. Available from: www.fi mea.fi /documents/542809/838272/29197_Biosimilaarien_vaihtokelpoisuus_EN.pdf
8. GaBI Online - Generics and Biosimilars Initiative. Saving money and building trust in biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 May 26]. Available from: www.gabionline.net/Reports/Saving-money-and-building-trust-in-biosimilars
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2017 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment