Estimates for the potential savings from the use of infliximab biosimilars in Canada suggests that savings by 2021 could be between CA$447 million and CA$2,310 million, depending on the level of uptake.
Biosimilar infliximab could save Canada over CA$2 million by 2021
Home/Reports
|
Posted 04/09/2020
 0
Post your comment
0
Post your comment

In a follow up to a report on the uptake of biosimilar infliximab in Canada [1], the Patented Medicine Prices Review Board (PMPRB) has published projected cost savings from the use of biosimilar infliximab under different scenarios.
Infliximab is a monoclonal antibody treatment for autoimmune diseases including inflammatory bowel disease, rheumatoid arthritis and psoriasis. Infliximab was one of the first biosimilar medicines to be approved in Canada. Pfizer’s infliximab biosimilar Inflectra was first sold in the country in 2015. The market for infliximab in Canada is currently worth CA$1 billion, according to the PMPRB.
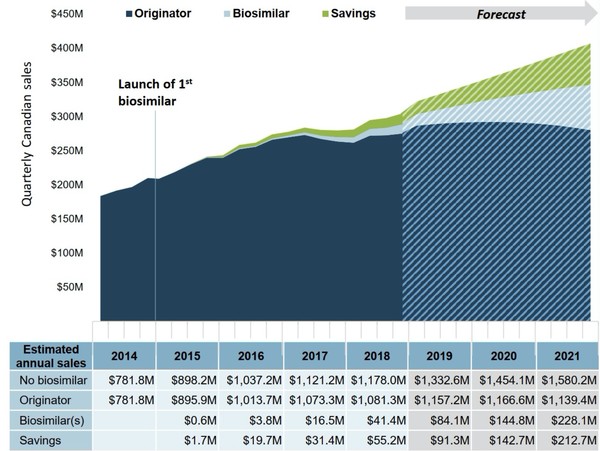
Between 2015 and 2018 in Canada, CA$62 million was spent on the biosimilar, resulting in savings of approximately CA$108 million. If the same trend continues, savings from 2019 until the end of 2021 are expected to be CA$447 million, see Figure 1.
Figure 1: Estimated cost savings for infliximab biosimilars based on current Canadian trends
Data is based on quarterly Canadian sales from 2015 to 2018 and forecast to 2021.
Source: IQVIA MIDAS database, prescription retail and hospital markets.
However, the PMPRB reports that if uptake of biosimilar infliximab increased to the median Organisation for Economic Co-operation and Development (OECD) level, savings could increase by almost CA$1 million to CA$1,412 million in total. This is based on total sales of CA$1,644 million.
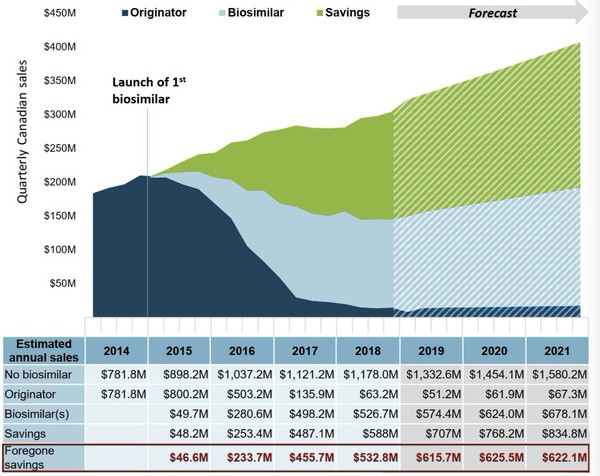
In the ‘OECD high scenario’, if uptake matched the median of the top four OECD countries and the biosimilar price was the same as the OECD median, sales between 2019 and 2021 are forecasted to be CA$1,876 million with savings of CA$2,310 million, see Figure 2 – over CA$1.8 million more than the current trend predicts.
Figure 2: Estimated cost savings for infliximab biosimilars in OECD high scenario
Based on quarterly sales under the OECD high scenario from 2015 to 2018 and forecast to 2021.
Source: IQVIA MIDAS database, prescription retail and hospital markets.
These findings demonstrate the high potential cost savings from the use of infliximab biosimilars in Canada if uptake were increased. Data shows uptake for biosimilar infliximab was less than 10% in 2018 [1], which suggests further initiatives to improve market penetration are necessary.
Related articles
Biosimilar infliximab reduces medication costs by two thirds in Finland
Low levels of biosimilar uptake in Canada
Use of biosimilar infliximab gives savings for patients
Reference
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilar infliximab uptake in Canada [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Sep 4]. Available from: www.gabionline.net/Reports/Biosimilar-infliximab-uptake-in-Canada
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Source: Government of Canada
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment