During the period of 2013 to 2015, biosimilars has been increasingly on the agenda around the world. Biosimilars are making steady progress, as can be seen by the increasing number of events, including approvals for biosimilars around the world [1].
Biosimilar events from 2013 to 2015
Home/Reports
|
Posted 19/06/2015
 0
Post your comment
0
Post your comment
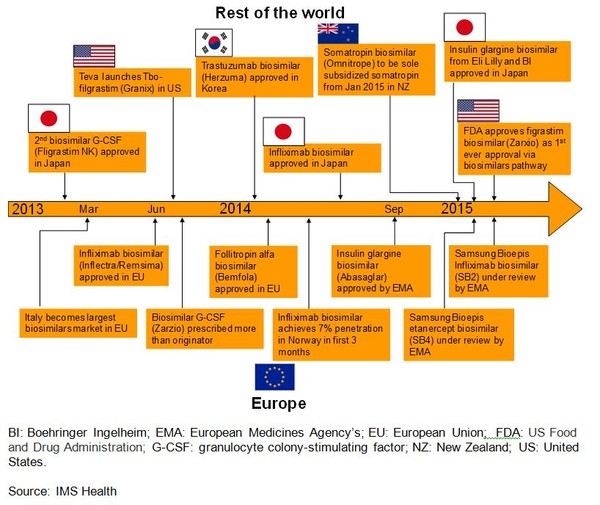
Once again, Europe is leading the way with the approval of the first monoclonal antibody biosimilar, and the US finally approved its first biosimilar. Some of the major biosimilars events that have taken place in 2013 to 2015 are shown in Figure 1.
Figure 1: Biosimilars events in the period 2013 to 2015
Japan approved its second biosimilar granulocyte colony-stimulating factor (G-CSF), Filgrastim NK, produced by Nippon Kayaku and development partner Teva Pharmaceutical Industries (Teva) in February 2013 [2]. South Korean biotechnology company Celltrion announced on 4 July 2014 that the company had received marketing approval for its infliximab biosimilar, Remsima, in Japan [3]. Eli Lilly and Boehringer Ingelheim confirmed on 19 January 2015 that they had received Japanese regulatory approval for their biosimilar insulin glargine product (LY2963016) [4].
South Korea approved a trastuzumab biosimilar, Herzuma, in January 2014 [5].
Once again, Europe has been leading the way with the landmark decision on 27 June 2013 by the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) to recommend approval of the infliximab biosimilar (Inflectra/Remsima), proving that the biosimilar concept can be successfully applied to such complex molecules as monoclonal antibodies [6].
In January 2014, EMA’s CHMP also recommended the granting of a marketing authorization for biosimilar infertility treatment Bemfola (follitropin alfa) [7]. This was followed by EMA’s CHMP announcing on 27 June 2014 that it had recommended granting of marketing authorization for a biosimilar insulin glargine product [Abasaglar (LY2963016)] [8]. In March 2015, South Korean electronics giant Samsung and biotechnology company Biogen Idec’s joint venture Samsung Bioepis announced that its infliximab biosimilar candidate, SB2, had been accepted for review by EMA [9].
And perhaps the most significant event was the US Food and Drug Administration’s approval of Sandoz’s biosimilar, Zarxio (filgrastim-sndz), the first biosimilar ever to be approved in the US, on 6 March 2015 [10].
Editor’s Comment
If you would like to receive a high resolution copy of Figure 1 please send us an email.
*For profit organizations subjected to a fee
Related articles
Biosimilars penetration in Europe varies widely
Cost reductions due to biosimilars in Europe
Biosimilars applications under review by EMA – April 2015
Biosimilar events from 2012 to 2014
Biosimilars approved in Europe
References
1. Rickwood S. Perspectives on the evolving biosimilars landscape. 13th EGA-European Biosimilars Group Conference; 23-24 April 2015; London, UK.
2. GaBI Online - Generics and Biosimilars Initiative. Japan approves second biosimilar G-CSF [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/Japan-approves-second-biosimilar-G-CSF
3. GaBI Online - Generics and Biosimilars Initiative. Biosimilar infliximab receives approval in Japan and Turkey [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/Biosimilar-infliximab-receives-approval-in-Japan-and-Turkey
4. GaBI Online - Generics and Biosimilars Initiative. Japanese approval for insulin glargine biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/Japanese-approval-for-insulin-glargine-biosimilar
5. GaBI Online - Generics and Biosimilars Initiative. Biosimilar trastuzumab approved in Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/Biosimilar-trastuzumab-approved-in-Korea
6. GaBI Online - Generics and Biosimilars Initiative. EC approves first monoclonal antibody biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/EC-approves-first-monoclonal-antibody-biosimilar
7. GaBI Online - Generics and Biosimilars Initiative. EMA approves follitropin alfa biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/EMA-approves-follitropin-alfa-biosimilar
8. GaBI Online - Generics and Biosimilars Initiative. EMA approves biosimilar insulin [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/EMA-approves-biosimilar-insulin
9. GaBI Online - Generics and Biosimilars Initiative. Samsung Bioepis submits second biosimilar to EMA [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/Samsung-Bioepis-submits-second-biosimilar-to-EMA
10. GaBI Online - Generics and Biosimilars Initiative. FDA approves its first biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-its-first-biosimilar
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2015 Pro Pharma Communications International. All Rights Reserved.
Source: IMS Health
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment