During her presentation at the European Commission’s Multi-Stakeholder Conference, which was held in Brussels, Belgium on 14 September 2018, Dr Elena Wolff-Holz discussed the importance of carrying out clinical trials with biosimilars [1].
Biosimilar clinical trials as confirmatory evidence
Home/Reports
|
Posted 05/04/2019
 0
Post your comment
0
Post your comment

Dr Wolff-Holz works at the Paul Ehrlich Institut, Federal Agency for Vaccines and Biomedicines and is Chair of the Biosimilar Medicinal Products Working Party (BMWP) of the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP).
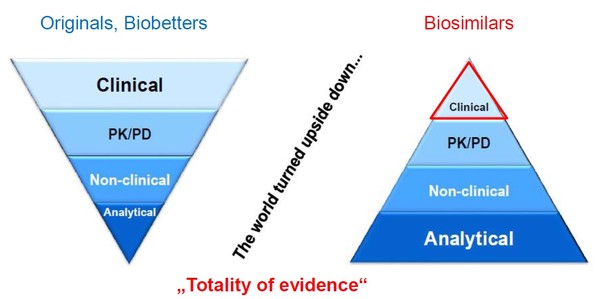
Her presentation highlighted how for biosimilars the world is turned upside down with respect to regulatory requirements for approval in the European Union (EU), see Figure 1.
Figure 1: Regulatory requirements for originator biologicals and biosimilars in the EU
EU: European Union; PD: pharmacodynamic; PK: pharmacokinetic.
Whereas for originator biologicals the onus is on clinical trials, for a biosimilar this is only a small part of the submission package. The onus is instead placed on proving analytical similarity with the originator biological.
For clinical trials of candidate biosimilars several criteria will determine ‘biosimilarity’. Sensitive attributes are evaluated with multiple complimentary methods and extrapolation may be allowed.
Dr Wolff-Holz gave several examples of clinical trials for biosimilars comparing rituximab biosimilars to MabThera and trastuzumab biosimilars to Herceptin.
I. Clinical comparability studies rituximab biosimilars versus MabThera
Truxima (CT-P10)
FDA: approved (November 2018 [2]); EMA: approved (February 2017 [3])
Study CT-P10 1.1 and extension study CT-P10 1.3 in patients with rheumatoid arthritis
• 2-arm, 72 week follow up, n = 154
• Pharmacokinetic (PK) (primary endpoint), pharmacodynamic (PD), efficacy and safety (secondary endpoints) of Truxima vs MabThera
Study CT-P10 3.2 in patients with rheumatoid arthritis
• 3-arm, 76 week follow up, n = 372 patients
• (Part 1) PK of Truxima vs Rituxan and MabThera (primary endpoint)
• (Part 2) Efficacy of Truxima vs Rituxan and MabThera (primary endpoint) PK, PD, Safety, Efficacy of Truxima vs Rituxan (secondary endpoints, Parts 1 & 2)
Study CT-P10 3.3 (supportive) in patients with advanced follicular lymphoma (AFL)
• 2-arm, 3 year follow up, n = 121
• (Part 1) PK of Truxima vs Rituxan (primary endpoint)
• (Part 2) Efficacy of Truxima vs Rituxan (non inferiority) (primary endpoint)
• Efficacy, PD, Safety of Truxima vs Rituxan (secondary endpoints, Parts 1 & 2)
Rixathon (GP2013)
FDA: pending approval; EMA: approved (June 2017 [4])
Study GP13-201 in patients with rheumatoid arthritis
• 2 arm, 52 week follow up, n = 173
• Pivotal PK (primary endpoint), PD (key secondary endpoint), safety and efficacy of Rixathon vs MabThera (Part 1), Rixathon vs Rituxan (Part 2)
Study GP13-301 (Pivotal) in patients with AFL
• 2 arms, follow up: 3 years
n = 627 patients induction, n = 462 patients maintenance
• Efficacy, safety and PK/PD of Rixathon vs MabThera in combination with other therapies followed by maintenance therapy
II. Clinical comparability studies trastuzumab biosimilars versus Herceptin
Ogivri (MYL 14010)
FDA: approved (December 2017 [5]); EMA: approved (December 2018 [6])
Study MYL-Her-1002 in healthy volunteers
• 3-arm, 72 week follow up, n = 120 patients
• PK (primary endpoint)
No data in neoadjuvant indication
No data in adjuvant indication
No data in gastric cancer indication
Study MYL-Her-3001 in combination with taxane in previously untreated patients with metastatic breast cancer
• 2-arm, 48 week follow up, n = 493 patients
• Efficacy: Primary endpoint: objective response rate (ORR)
• Safety and immunogenicity
Ontruzant (SB3)
FDA: approved (January 2019 [7]); EMA: approved (November 2017 [8])
Study NCT02075073 in healthy volunteers
• 3 arm, 52 weeks follow up, n = 109 patients
• PK primary endpoint (published)
Study NCT02149524 in patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer in neoadjuvant setting
• 2 arms, follow up: 3 years n = 806 patients
• Efficacy: Primary endpoint: total pathological complete response (tpCR); Secondary endpoints: event-free survival (EFS), overall survival (OS)
• Safety and immunogenicity
No data in metastatic breast cancer indication
No data in adjuvant indication
No data in gastric cancer indication
Dr Wolff-Holz concluded that the ‘EU is a highly regulated territory with high market entry barriers for biosimilars’. She added that ‘clinical trials are models to study comparability, confirmatory nature, different development approaches possible for same active substance’.
Conflict of interest
The author of the presentation [1] stated that the views presented in the presentation were her own and do not necessarily reflect the views of the Paul-Ehrlich-Institut or EMA.
Related article
Use of biosimilars in oncology in Europe
References
1. Wolff-Holz E. Biosimilars use in oncology: overview by the European Medicines Agency on biosimilars approved so far. EC Multi-Stakeholder Conference; 14 September 2018; Brussels, Belgium.
2. GaBI Online - Generics and Biosimilars Initiative. FDA approves first rituximab biosimilar Truxima [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Apr 5]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-first-rituximab-biosimilar-Truxima
3. GaBI Online - Generics and Biosimilars Initiative. EC approval for first cancer biosimilar Truxima [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Apr 5]. Available from: www.gabionline.net/Biosimilars/News/EC-approval-for-first-cancer-biosimilar-Truxima
4. GaBI Online - Generics and Biosimilars Initiative. EC approval for rituximab biosimilar Rixathon [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Apr 5]. Available from: www.gabionline.net/Biosimilars/News/EC-approval-for-rituximab-biosimilar-Rixathon
5. GaBI Online - Generics and Biosimilars Initiative. FDA approves trastuzumab biosimilar Ogivri [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Apr 5]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-trastuzumab-biosimilar-Ogivri
6. GaBI Online - Generics and Biosimilars Initiative. Mylan gains nod for Ogivri and launches Hulio in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Apr 5]. Available from: www.gabionline.net/Biosimilars/News/Mylan-gains-nod-for-Ogivri-and-launches-Hulio-in-Europe
7. GaBI Online - Generics and Biosimilars Initiative. FDA approves trastuzumab biosimilar Ontruzant [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Apr 5]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-trastuzumab-biosimilar-Ontruzant
8. GaBI Online - Generics and Biosimilars Initiative. EC approval for trastuzumab biosimilar Ontruzant [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Apr 5]. Available from: www.gabionline.net/Biosimilars/News/EC-approval-for-trastuzumab-biosimilar-Ontruzant
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2019 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment