The landscape of blockbuster medicines in Europe has been changing over the last years from small molecule drugs to biologicals.
Biologicals dominate Europe’s best sellers
Home/Reports
|
Posted 27/06/2014
 2
Post your comment
2
Post your comment

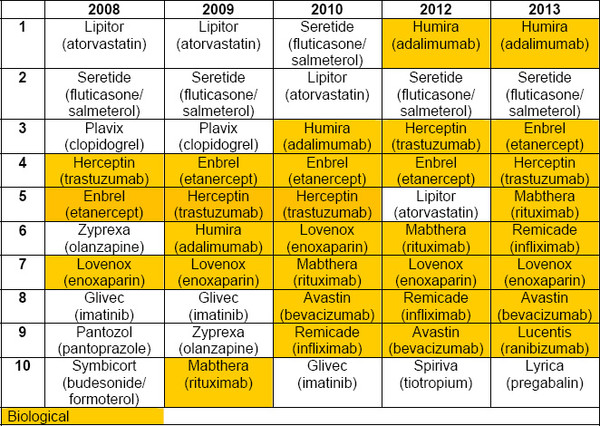
Biologicals now dominate the top 10 list of best-selling medicines in Europe, with eight of the top 10 best-selling drugs now being biologicals. This compares to only three biologicals that made the top 10 list back in 2008, see Table 1.
Table 1: Top 10 best-selling drugs in Europe 2008–2013
Source: IMS Health, MIDAS, MAT June 2013
The patents on all of these blockbuster biologicals have already expired or expire by 2022 at the latest [1], making them targets for biosimilars developers.
There are also more than 200 new biologicals in the pipeline (phase II to be registered), all of which could also be future targets for biosimilars [2].
Related article
The global biologicals market
References
1. GaBI Online - Generics and Biosimilars Initiative. US$67 billion worth of biosimilar patents expiring before 2020 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Jun 27]. Available from: www.gabionline.net/Biosimilars/General/US-67-billion-worth-of-biosimilar-patents-expiring-before-2020
2. GaBI Online - Generics and Biosimilars Initiative. Biotech pipeline and biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2014 Jun 27]. Available from: www.gabionline.net/Biosimilars/Research/Biotech-pipeline-and-biosimilars
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2013 Pro Pharma Communications International. All Rights Reserved.
Posted 05/08/2014 by Justyna K, GaBI Online Editorial Office
Response to 'Top 10 best-selling drugs in Europe 2008–2013'
Dear Mr Kashi, Thank you for your comment. We have also noticed this error and subsequently updated the article. Please continue with your valuable feedback. Kind regards, Justyna
Posted 29/06/2014 by Ramesh Kashi
Top 10 best-selling drugs in Europe 2008–2013
Lipitor (column 2012 / row 5) in Table 1 is not a biologic (high-lighted in yellow)
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment