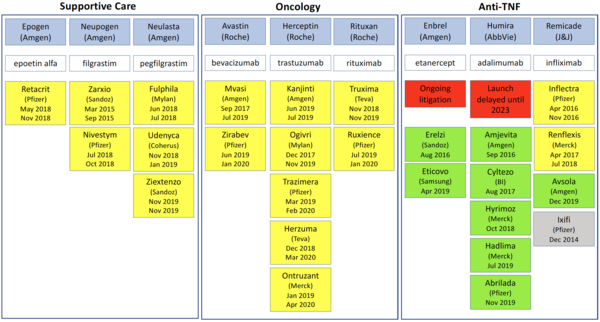
To date (19 June 2020), the US Food and Drug Administration (FDA) has approved 27 biosimilars, plus four follow-on biologicals [1]. The pipeline for biosimilars continues to grow, however, of the 27 biosimilars approved, only 17 have so far been launched [2].
Approval and launch dates for US biosimilars
Home/Reports
|
Posted 19/06/2020
 0
Post your comment
0
Post your comment

In fact, biosimilars currently make up only 2.3% of the US biologicals marketplace. Currently, 90% of global biosimilars sales take place in Europe, despite 60% of overall biologicals sales occurring in the US [3].
Reasons for delays in launching biosimilars and the sluggish biosimilars market in the US may include anti-competitive behaviours and other market and regulatory dynamics that currently discourage market uptake of biosimilars in the US [3].
In Europe, biosimilars are usually launched soon after approval and immediately after the patent expiry dates of the originator biologicals. However, in the US this is often not the case, with launch dates coming years after FDA approval in some cases, see Table 1.
One clear example of an originator company delaying the launch of biosimilars in the US is that of biosimilars to the adalimumab biological Humira. AbbVie brought patent litigation against a whole host of biosimilars makers and eventually made agreements with these companies that would delay the launch of adalimumab biosimilars until June 2023 at the earliest [4].
In contrast, in Europe the first adalimumab biosimilars, Amgevita and Solymbic, were approved (and launched) in March 2017 [5] and since then eight more adalimumab biosimilars have been approved in the region [6].
Another case is that of the etanercept biological Enbrel. Although Sandoz, a division of Novartis, had its biosimilar Erelzi (etanercept-szzs) approved by FDA back in 2016 [7] it is still to launch the product due to ongoing patent litigation [8].
Again, in Europe, the contrast is clear, with etanercept biosimilars Benepali and Erelzi being approved (and launched) in January 2016 and June 2017, respectively [6].
Biosimilars on average can cost 30% less than reference biologicals, with the potential to save the US up to US$71 billion in the next decade. In fact, the US could save US$7.2 billion annually if the biosimilars marketplace grew to 75%, and even more savings could be realized if more classes of biosimilars are approved.
According to Juliana Reed, President of the Biosimilars Forum, the only way for the US to change the current situation in the US ‘is to implement measures to support a viable US biosimilars market’ [3].
Table 1: Approval and launch dates for biosimilars in the US
BI: Boehringer Ingelheim; J&J: Johnson and Johnson; TNF: tumour necrosis factor.
Source: AmerisourceBergen
Related articles
AbbVie makes more deals delaying adalimumab biosimilars in the US
AbbVie and Coherus sign licensing deal for Humira biosimilar
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jun 19]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-the-US
2. McGowan S, Jesse M. Biosimilars Pipeline Report. AmerisourceBergen, May 2020.
3. GaBI Online - Generics and Biosimilars Initiative. The sluggish US biosimilars market [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jun 19]. Available from: www.gabionline.net/Reports/The-sluggish-US-biosimilars-market%20
4. GaBI Online - Generics and Biosimilars Initiative. Boehringer Ingelheim finally signs licensing deal for Humira biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jun 19]. Available from: www.gabionline.net/Pharma-News/Boehringer-Ingelheim-finally-signs-licensing-deal-for-Humira-biosimilar
5. GaBI Online - Generics and Biosimilars Initiative. EMA approval for adalimumab biosimilars Amgevita and Solymbic [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jun 19]. Available from: www.gabionline.net/Biosimilars/News/EMA-approval-for-adalimumab-biosimilars-Amgevita-and-Solymbic
6. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jun 19]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
7. GaBI Online - Generics and Biosimilars Initiative. FDA approves biosimilar etanercept Erelzi [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Sep 19]. Available from: www.gabionline.net/Biosimilars/News/FDA-approves-biosimilar-etanercept-Erelzi
8. GaBI Online - Generics and Biosimilars Initiative. Court rules Amgen’s patents on Enbrel are valid, Sandoz to appeal [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jun 19]. Available from: www.gabionline.net/Biosimilars/General/Court-rules-Amgen-s-patents-on-Enbrel-are-valid-Sandoz-to-appeal
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Source: AmerisourceBergen
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets









Post your comment