According to the US Food and Drug Administration (FDA) guideline on ‘Scientific Considerations in Demonstrating Biosimilarity to a Reference Product’ [1], ‘FDA expects that first, a sponsor will extensively characterize the proposed product and the reference product with state‐of‐the‐art technology, because extensive characterization of both products serves as the foundation for a demonstration of biosimilarity’ [2].
Analytical consideration in demonstrating similarity for biosimilars
Home/Reports
|
Posted 13/07/2018
 0
Post your comment
0
Post your comment

The guidelines also specify that biosimilar applicants should demonstrate biosimilarity using a stepwise approach, which includes structural and functional characterization, animal toxicity, pharmacokinetics and pharmacodynamics, immunogenicity, and clinical safety and effectiveness [2].
The stepwise approach starts with extensive structural and functional characterization. This allows for an evaluation of the analytical differences and resulting residual uncertainty about biosimilarity. The next steps to try to address that uncertainty can then be identified. Orthogonal methods can then be used to further evaluate the impact on function. Control strategies should then be used to minimize differences. Finally, non-clinical and clinical evaluation of the biosimilarity should be carried out.
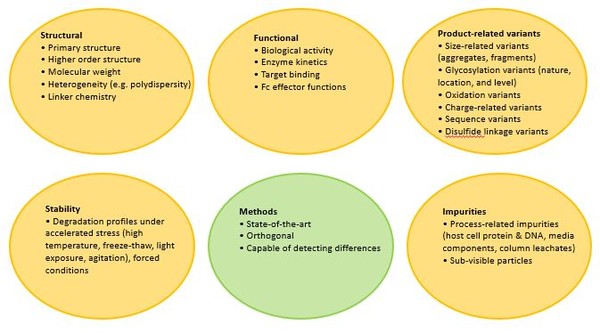
In a presentation on Expectations and Approaches for Demonstrating Analytical Similarity at the US Drug Information Association’s Biosimilars Conference [3], Dr Lynch highlighted that demonstrating analytical similarity is the foundation to demonstrating biosimilarity. Analytical considerations that need to be taken into account are given in Figure 1.
Figure 1: Analytical considerations for biosimilarity
Disclaimer
Dr Patrick Lynch, Product Quality Reviewer at the US Food and Drug Administration (FDA), stated that his presentation reflects the views of the author and should not be construed to represent FDA’s views or policies.
Related articles
Addressing uncertainty in biosimilarity
Lot selection and handling for biosimilarity
Ranking and evaluation of quality attributes for biosimilarity
Analytical similarity for biosimilars
References
1. GaBI Online - Generics and Biosimilars Initiative. US guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Apr 26]. Available from: www.gabionline.net/Guidelines/US-guidelines-for-biosimilars
2. U.S. Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. April 2015 [homepage on the Internet]. [cited 2018 Jul 13]. Available from: www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf
3. Lynch P. Expectations and approaches for demonstrating analytical similarity. DIA Biosimilars Conference; 24-25 October 2017; Bethesda, Maryland, USA.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2018 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment