The Centers for Medicare and Medicaid Services (CMS) released a memorandum that acts as a new guidance for the Medicare Drug Price Negotiation Program [1].
New guidance for Medicare Drug Price Negotiation Program
Home/Policies & Legislation
|
Posted 20/04/2023
 0
Post your comment
0
Post your comment

Issued on 15 March 2023, this is initial guidance regarding implementation of sections 11001 and 11002 of the Inflation Reduction Act (IRA) (P.L. 117-169), which establishes the Medicare Drug Price Negotiation Program to negotiate maximum fair prices (MFPs) for certain high expenditure, single source drugs and biological products.
Under the Negotiation Program, the government will select 10 Part D drugs for negotiation for initial price applicability year 2026, and scale up to 20 Part B and Part D drugs by 2028. The guidance outlines the initial steps that CMS will take to implement the programme.
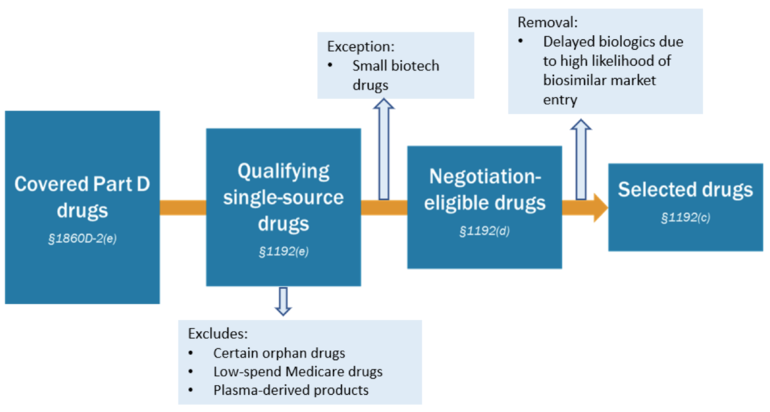
Overall, the guidance provides insights into the following aspects of the CMS's plan for drug negotiation in 2026: the selection process for drugs up for negotiation, see Figure 1, the qualifications for manufacturers whose drugs are selected, the contractual and data submission processes for manufacturers in negotiations with CMS, considerations for negotiation with manufacturers, the negotiation process itself, including initial offers, counteroffers, and post-counteroffer procedures, and the protocols for removing drugs from CMS's list. The plan also defines MFP eligible individuals, outlines CMS's methods for monitoring manufacturer compliance with the Negotiation Program, including the use of excise taxes and monetary penalties, specifies that Medicare Part D plans must include covered drugs on their formularies during the effective period of the MFP, and explains how the Part B and Part D Prescription Drug Rebate Programs will be applied to selected drugs.
Figure 1: Diagram of process for selecting drugs for negotiation for initial price applicability year 2026
Another key aspect of the programme is the creation of a formulary, or a list of prescription drugs that will be covered by Medicare. The formulary will be based on clinical effectiveness and value, and will prioritize drugs that provide the greatest benefit to Medicare beneficiaries. The CMS guidance states that the formulary will be updated annually to reflect new drugs and changes in clinical evidence.
The guidance also addresses concerns that drug manufacturers may refuse to negotiate with CMS. The Secretary of Health and Human Services is authorized to impose monetary penalties on manufacturers that refuse to participate in negotiations or fail to meet established pricing levels. The guidance notes that any penalties will be designed to incentivize compliance with the programme and minimize disruptions in patient access to needed medications.
The CMS notes in the guidance that the programme is intended to promote competition and improve access to affordable medications for Medicare beneficiaries. In addition, the programme hopes to save billions of dollars in healthcare costs over the next decade. As the programme moves forward, it will be important to monitor its impact on patient access to medications and overall healthcare costs.
CMS sought comments on various aspects of its Negotiation Program guidance, due by 12 April 2023.
The memo notes that CMS is not following the typical notice-and-comment requirements of the Administrative Procedure Act or the Medicare statute, citing both legislative direction and its own assessment: ‘CMS finds that notice and public procedure on this guidance would be impracticable, unnecessary, and contrary to the public interest’.
‘To undertake such a major change to the Medicare program outside of the notice-and-comment period is highly unusual and runs counter to long-established procedures’, says ASBM Executive Director Mr Michael Reilly, who served for 6 years in the Office of the Secretary at the Department of Health and Human Services (HHS) as Medicare Part D was being developed and implemented. ‘Unfortunately, rushed policy made absent stakeholder input rarely results in good policy’.
Related articles
United States legislation to improve access to insulin
Biosimilars policy considerations in the US
FDA releases new patient guidance on biosimilars
Economic considerations for biosimilars in the US
|
LATIN AMERICAN FORUM View the latest headline article: El Grupo Español de Psoriasis actualiza su posicionamiento sobre los biosimilares Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: El Grupo Español de Psoriasis actualiza su posicionamiento sobre los biosimilares !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
Reference
1. Department of Health & Human Services. Centers for Medicare & Medicaid Services. Medicare Drug Price Negotiation Program: Initial Memorandum, Implementation of Sections 1191 – 1198 of the Social Security Act for Initial Price Applicability Year 2026, and Solicitation of Comments [homepage on the Internet]. [cited 2023 Apr 14]. Available from: www.cms.gov/files/document/medicare-drug-price-negotiation-program-initial-guidance.pdf
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.
Source: CMS
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
China updates regulations to encourage research and innovation and improved drug safety
Brazil and Mexico forge alliance to streamline medical approvals and boost production
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
China updates regulations to encourage research and innovation and improved drug safety

Home/Policies & Legislation Posted 04/03/2026
Brazil and Mexico forge alliance to streamline medical approvals and boost production

Home/Policies & Legislation Posted 09/02/2026
EU accepts results from FDA GMP inspections for sites outside the US

Home/Policies & Legislation Posted 27/01/2026
WHO to remove animal tests and establish 17 reference standards for biologicals

Home/Policies & Legislation Posted 07/01/2026
The best selling biotechnology drugs of 2008: the next biosimilars targets






Post your comment