Under the Global Gateway umbrella, the EU and Latin America and the Caribbean (EU-LAC) are partnering on health resilience and vaccine production, and as part of this effort, they organized a high-level pharma forum and matchmaking event – the European Union - Latin America and Caribbean Summit – from February to April 2023.
EU and Latin America/Caribbean launch virtual marketplace for pharmaceuticals
Home/Policies & Legislation
|
Posted 31/03/2023
 0
Post your comment
0
Post your comment
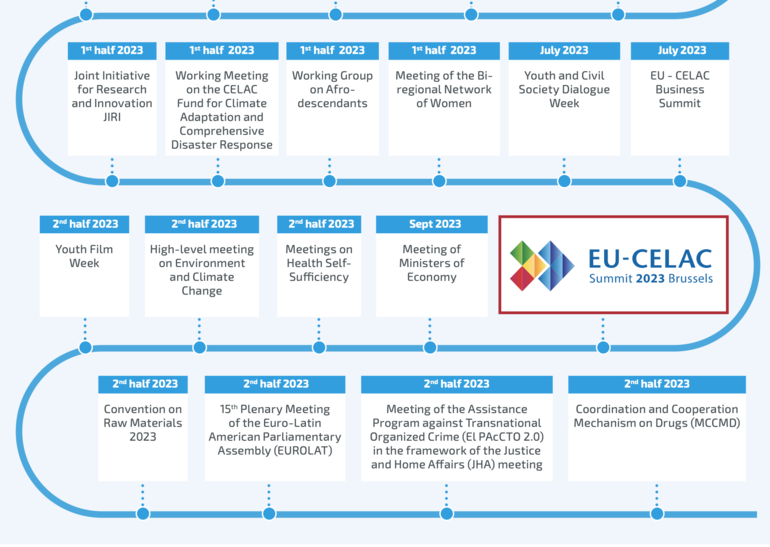
The event aims to promote collaboration between pharmaceutical industries in both regions, and it is a component of the CELAC (Community of Latin American and Caribbean States)-EU Bi-Regional Roadmap 2022–2023, see below roadmap in 2023.
Diversification of global pharmaceutical production is essential for global health security and the EU is keen to contribute to the creation of ecosystems conducive to ensuring equitable access to quality health products.
There is a significant opportunity for cooperation between companies and important healthcare organizations in Latin America, the Caribbean and Europe. An operational roadmap has been developed containing several areas for engagement:
- private sector engagement to strengthen the region’s vaccine manufacturing capacity
- improved regional supply chains, trade integration and business environment
- regulatory strengthening
- demand consolidation and pooled procurement
- scientific cooperation
- pandemic preparedness and health security.
The EU-LAC partnership on health resilience and vaccine production is aimed at boosting local manufacturing capacities for vaccines, medicines and health technologies, as well as strengthening health systems resilience.
To support the above objective, the European Commission organized a (virtual) marketplace and matchmaking event on pharmaceuticals from 2 February to beginning of April 2023, and a high-level forum on 21 March 2023.
The marketplace and matchmaking event consisted of an online platform where interested parties were able register and to identify potential partners and meet their counterparts through confidential Business-to-Business (B2B) sessions.
The EU-LAC pharma matchmaking initiative concluded with a high-level forum in Brussels on 21 March 2023 with the theme ‘Sharing pharmaceutical innovations between the European Union and Latin America and the Caribbean’. The scope of the forum from a products perspective included therapeutics, vaccines, generics and biosimilars. The aim was to identify synergies that meet the public health needs of Latin America and the Caribbean and EU markets.
Pharmaceutical innovations, together with higher living standards and more effective public health interventions, have been significantly important to extend life expectancy and improve population quality of life in Latin America and the Caribbean [2].
Related articles
EU public consultation on revised framework for compulsory licensing of patents
Pharmaceutical manufacturing companies in Brazil
Pharmaceutical companies in Argentina
Market outlook for biological medicines in Brazil 2016‒2025
|
LATIN AMERICAN FORUM View the latest headline article: La UE y América Latina/Caribe lanzan un mercado virtual de productos farmacéuticos Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: La UE y América Latina/Caribe lanzan un mercado virtual de productos farmacéuticos !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
Reference
1. GaBI Online - Generics and Biosimilars Initiative. Pharmaceutical companies in Latin America and the Caribbean [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Mar 31]. Available from: www.gabionline.net/generics/general/pharmaceutical-companies-in-latin-america-and-the-caribbean
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.
Source: European Commission
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
China updates regulations to encourage research and innovation and improved drug safety
Brazil and Mexico forge alliance to streamline medical approvals and boost production
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
China updates regulations to encourage research and innovation and improved drug safety

Home/Policies & Legislation Posted 04/03/2026
Brazil and Mexico forge alliance to streamline medical approvals and boost production

Home/Policies & Legislation Posted 09/02/2026
EU accepts results from FDA GMP inspections for sites outside the US

Home/Policies & Legislation Posted 27/01/2026
WHO to remove animal tests and establish 17 reference standards for biologicals

Home/Policies & Legislation Posted 07/01/2026
The best selling biotechnology drugs of 2008: the next biosimilars targets






Post your comment