There finally appears to be some light at the end of the tunnel, with one of the main victims of shortages—the sterile injectable drug propofol—finally being declared no longer in short supply by FDA.
Some relief from drug shortages in the US
Home/Pharma News
|
Posted 10/11/2011
 0
Post your comment
0
Post your comment

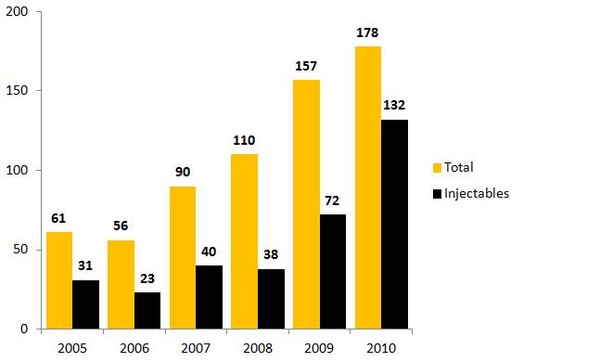
The number of drug shortages in the US has tripled in the last six years and is now affecting all areas of healthcare. In 2010, 178 drug shortages were reported by the FDA to be in short supply, 74% (132) of which involved sterile injectable drugs [1], see Figure 1 [2]. The shortages affected cancer drugs, anaesthetics and IV electrolytes, as well as drugs needed for emergency medicine. Worse still, many of the drugs identified in 2010 remain unavailable or in short supply in 2011.
Figure 1: Drug shortages reported to FDA in the US 2005–2010
Source: FDA
The shortages are at record high levels and, while regulators blame quality and manufacturing problems for the crisis, others blame the FDA’s own policies for contributing to the problem.
Good manufacturing practice rules, the agency’s Unapproved Drugs Initiative and a lack of FDA inspectors have all been named as factors causing drug shortages [3].
Health providers on the other hand say that the companies who make these drugs, long sold in generic form, have a diminishing interest in ensuring a strong supply. Only five to seven companies produce 80% of the medicines in short supply, and stricter reimbursement policies have cut into the profits.
Despite all the doom and gloom and the fact that drug shortages are only expected to increase in 2011, there is some good news to shout about. APP Pharmaceuticals and Hospira reported that shortages of cytarabine and propofol had been resolved.
The propofol sedative has been in short supply since back in 2009 when manufacturers Teva Pharmaceuticals and Hospira were the subject of FDA recalls. While the leukemia treatment cytarabine—just one of the many cancer drugs experiencing shortages—has been in short supply for about a year.
In an attempt to alleviate the drug shortages President Barack Obama issued an executive order on 31 October 2011 in which he broadened reporting requirements for potential shortages. The order also instructed FDA to accelerate reviews of new generic drug applications and to provide information to the Justice Department about possible collusion or price gouging related to the shortages [4].
Editor’s Comment
Will such legislation help alleviate the drug shortage crisis? How can such drug shortages be avoided in the future? Are the standards for medicines manufacturing contributing to these shortages or are drug companies just concentrating on the big earners?
Please feel free to share your thoughts via email to editorial@gabionline.net or in the comments section below. What do you think are the reasons for drug shortages? Who is really to blame? How can the generics/biosimilars industry help to alleviate these shortages?
Related articles
Row continues over drug shortages
US drug shortages prompt oncologists to go into drug manufacture
US drug shortages – FDA may hinder, but APP may help
References
1. GaBI Online - Generics and Biosimilars Initiative. US drug shortages prompt oncologists to go into drug manufacture [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2011 November 10]. Available from: www.gabionline.net/Generics/News/US-drug-shortages-prompt-oncologists-to-go-into-drug-manufacture
2. A review of FDA’s approach to medical product shortage. US FDA; 2011 Oct 31; USA.
3. GaBI Online - Generics and Biosimilars Initiative. US drug shortages – FDA may hinder, but APP may help [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2011 November 10]. Available from: www.gabionline.net/Generics/News/US-drug-shortages-FDA-may-hinder-but-APP-may-help
4. GaBI Online - Generics and Biosimilars Initiative. Obama’s fresh attempts to ease drugs shortages in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2011 November 10]. Available from: www.gabionline.net/Pharma-News/Obama-s-fresh-attempts-to-ease-drugs-shortages-in-the-US
Source: FDA, Reuters
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
Formycon signs new aflibercept biosimilar pacts and launches ranivisio in Europe

Home/Pharma News Posted 13/11/2025
Bio-Thera and Stada expand biosimilars alliance to include tocilizumab

Home/Pharma News Posted 20/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment