Japanese pharmaceutical firms Gene Techno Science and Kishi Kasei announced on 2 December 2019 an agreement for the joint development of an aflibercept biosimilar, a treatment for macular degeneration to rival the reference drug Eylea.
Japanese firms to collaborate on Eylea biosimilar
Home/Pharma News
|
Posted 10/01/2020
 0
Post your comment
0
Post your comment
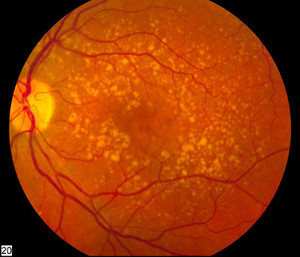
Aflibercept is a biological drug used in the treatment of macular degeneration, which occurs with age and causes vision loss. Macular degeneration is characterized by the growth of abnormal blood vessels in the eye. The treatment works by inhibiting vascular endothelial growth factor (VEGF), which causes the growth of new blood vessels.
It has been sold in the US and Europe under the brand name Eylea and has a market size in Japan estimated at Yen 60 billion (US$552 million).
The agreement also involves Fuso Pharmaceuticals, who have developed an aflibercept-producing cell line, which is expected to reduce manufacturing costs with its high yield.
Kishi Kasei manufactures colouring used in foods, cosmetics and medicines but has also been developing drug substances for biologicals. Under the agreement they will build the manufacturing system for the aflibercept biosimilar.
Gene Techno Science are still looking for a partner to promote drug development, non-clinical and clinical studies, marketing approval and sales in order to commercialize the product in Japan.
The agreement follows the news that South Korean firm Samsung Bioepis has extended its agreement with Biogen for the commercialization of its own aflibercept biosimilar, currently named only as SB15 [1].
Related articles
Alteogen gains approval to start aflibercept biosimilar trial in Korea
Reference
1. GaBI Online - Generics and Biosimilars Initiative. Samsung Bioepis expands biosimilars agreement with Biogen [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Jan 10]. Available from: www.gabionline.net/Pharma-News/Samsung-Bioepis-expands-biosimilars-agreement-with-Biogen
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Source: Gene Techno Science
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
Formycon signs new aflibercept biosimilar pacts and launches ranivisio in Europe

Home/Pharma News Posted 13/11/2025
Bio-Thera and Stada expand biosimilars alliance to include tocilizumab

Home/Pharma News Posted 20/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment