Fresenius Kabi, the generics unit of healthcare giant Fresenius, announced on 19 August 2013 that it had set up a joint venture with Indonesia-based PT Soho Global Health, acquiring a 51% share in its subsidiary PT Ethica Industri Farmasi.
Fresenius Kabi sets up generics joint venture in Indonesia
Home/Pharma News
|
Posted 23/08/2013
 0
Post your comment
0
Post your comment
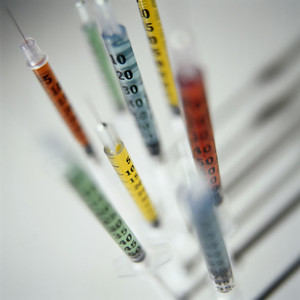
The joint venture will focus on generic intravenous drugs and infusion solutions and will make Fresenius Kabi the Indonesian market leader in intravenous generics.
Indonesia is an emerging country in the pharmaceutical business. Demand for healthcare, and thus prescription drugs, has been rising steadily. As a result of a universal healthcare programme, which is set to be introduced in 2014, most of the Indonesian population is expected to have access to modern healthcare by 2019.
As a result of these reforms to healthcare the Indonesian pharmaceutical market is expected to double to reach Euros 7.1 billion by 2018, making this an attractive deal for Fresenius Kabi.
The deal is expected to boost the product pipeline for PT Soho Global Health, while giving Fresenius Kabi access to valuable local manufacturing capabilities and a strong market presence. Fresenius Kabi also intends to use the joint venture as a hub for further expansion into the South East Asia Region.
The companies are expected to finalize the deal in the third quarter of 2013.
Related articles
Fresenius cashes in on drug shortages
Generics companies turn to ‘third tier’ countries
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2013 Pro Pharma Communications International. All Rights Reserved.
Source: Fresenius Kabi
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
Formycon signs new aflibercept biosimilar pacts and launches ranivisio in Europe

Home/Pharma News Posted 13/11/2025
Bio-Thera and Stada expand biosimilars alliance to include tocilizumab

Home/Pharma News Posted 20/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment