FDA Commissioner Ms Margaret Hamburg has reported that the FDA’s office of drug shortages has made progress in preventing drug shortages and working with industry to provide advanced warning when the agency is unable to prevent a shortage. In a posting on the agency’s FDA Voice Blog on 3 May 2012, Ms Hamburg said that she was ‘both amazed and delighted to see the progress that’s been made’.
FDA says efforts to prevent drug shortages are working
Home/Pharma News
|
Posted 11/05/2012
 0
Post your comment
0
Post your comment

An Executive Order was signed by President Barack Obama on 31 October 2011 in an attempt to address the escalating shortage of life-saving medicines in the US [1]. This was seen as an important step in bringing awareness to this critical public health issue and signalling the necessary tools and resources such as early notification and additional staff for FDA.
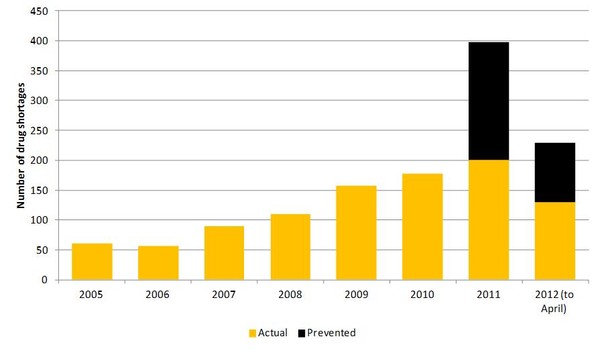
Since the signing of the Executive Order six months ago FDA reports that it has been able to prevent 128 drug shortages, and has had only 42 new drugs reported to be in short supply in 2012, compared to 90 new shortages during January to April 2011, see Figure 1. The agency also reports that it has managed to resolve shortages of methotrexate, a cancer drug used to treat childhood leukaemia.
FDA has also addressed the shortages of Doxil (liposomal doxorubicin), a drug used for ovarian cancer and other cancer regimens; and Leucovorin (folinic acid) injection, a cancer drug that is used along with methotrexate for children with a serious form of leukaemia; by allowing the temporary import of generics from India.
Figure 1: Number of drug shortages in the US by year
Source: FDA
FDA remains cautious about celebrating just yet, stating that, ‘drug shortages remain a serious, complex problem and the agency remains extremely concerned about all current and potential drug shortages.’ The US is not out of the woods yet despite recent successes in preventing drug shortages by FDA, the total number of drug shortages for 2012 still look to surpass those of 2011.
Related articles
Shortages of ADHD drugs expected during 2012
UK drug shortages are far from being solved
Cancer drug shortages in the US
Some relief from drug shortages in the US
Reference
1. GaBI Online - Generics and Biosimilars Initiative. Obama’s fresh attempts to ease drugs shortages in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 May 11]. Available from: www.gabionline.net/Pharma-News/Obama-s-fresh-attempts-to-ease-drugs-shortages-in-the-US
Source: FDA Blog
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
Formycon signs new aflibercept biosimilar pacts and launches ranivisio in Europe

Home/Pharma News Posted 13/11/2025
Bio-Thera and Stada expand biosimilars alliance to include tocilizumab

Home/Pharma News Posted 20/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment