On 15 October 2020, it was announced that two Essex Bio-Technology subsidiaries were entering an agreement with Shanghai Henlius Biotech. Under this, Essex Bio-Investment and Zhuhai Essex Bio-Pharmaceuticals will work with Henlius to co-develop a bevacizumab biosimilar for the treatment of ophthalmic diseases.
Essex and Henlius collaborate to deliver bevacizumab biosimilar for ophthalmic diseases
Home/Pharma News
|
Posted 06/11/2020
 0
Post your comment
0
Post your comment
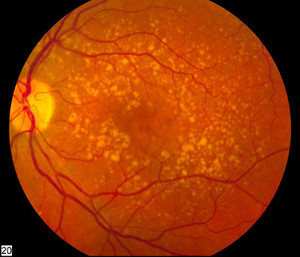
Bevacizumab is a monoclonal antibody and the originator, Avastin, is used to treat various cancers and age-related macular degeneration [1]. Although widely used for the treatment of ophthalmic conditions, bevacizumab has not been approved for this purpose globally.
The Essex and Henlius agreement hopes to be able to bring a bevacizumab biosimilar to ophthalmic disease patients across the world. There is a large market for such a product as there are over five million age-related macular degeneration patients in China and approximately 30 million globally. Their bevacizumab biosimilar will join that developed by other pharmaceutical companies, such as Samsung Bioepis and Biogen’s Aybintio [2] and Centus Biotherapeutics’ Equidacent [3, 4].
The bevacizumab copy biological has already been developed by Henlius and was granted an investigational new drug application (IND) for the indications of age-related macular degeneration and diabetic retinopathy in China. Henlius has submitted a patent for their new formulation of bevacizumab designed for ophthalmic use which potentially has better safety and stability. With this collaboration, Essex and Henlius will now conduct global multicentre clinical trials of the bevacizumab product in China, Australia, the European Union and the US, and subsequently apply for marketing authorization in around the globe.
Under the Co-development and Exclusive licence agreement, Henlius will undertake the preclinical and clinical development of bevacizumab. The Essex subsidiaries will be responsible for regulatory filings and commercialization. The development costs will be shared between the companies up to a total of US$30 million, with an 80:20 split being covered by Henlius and Essex, respectively. Should costs exceed this amount, a new agreement will be arranged. If Essex grants a third-party sub-licence for the commercialization of bevacizumab, Essex and Henlius will receive respective revenue shares of 80% and 20%. The agreement also states that Henlius is entitled to receive a signing payment, and further subsequent regulatory milestone payments for the initiation of the global clinical trial programme and the completion of the global clinical trial programme, together these will amount to US$25 million. As an additional reward for Henlius, Essex has agreed to make commercial milestone payments of up to US$18 million when the cumulative net sales generated by Essex from bevacizumab achieve specified milestones, and US$30 million for every net sales of US$1 billion above specified milestone. Henlius will also receive royalties at a progressive rate from 6% to 10% of the annual net sales of bevacizumab.
Related articles
Positive phase Ib results for TYVYT + bevacizumab copy biological IBI305
EU approves Henlius copy biologicals facility in China
Positive results for Bio-Thera’s bevacizumab copy biological
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars-of-bevacizumab [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Nov 6]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-of-bevacizumab
2. GaBI Online - Generics and Biosimilars Initiative. EMA approves bevacizumab and teriparatide biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Nov 6]. Available from: www.gabionline.net/Biosimilars/News/EMA-approves-bevacizumab-and-teriparatide-biosimilars
3. GaBI Online - Generics and Biosimilars Initiative. EMA recommends approval of bevacizumab biosimilar Equidacent [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Nov 6]. Available from: www.gabionline.net/Biosimilars/News/EMA-recommends-approval-of-bevacizumab-biosimilar-Equidacent
4. GaBI Online - Generics and Biosimilars Initiative. EC approval for bevacizumab biosimilar Equidacent [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Nov 6]. Available from: www.gabionline.net/Biosimilars/News/EC-approval-for-bevacizumab-biosimilar-Equidacent
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
Formycon signs new aflibercept biosimilar pacts and launches ranivisio in Europe

Home/Pharma News Posted 13/11/2025
Bio-Thera and Stada expand biosimilars alliance to include tocilizumab

Home/Pharma News Posted 20/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment