Repurposing and re-registering old generics is a cost-effective and time-efficient way of finding new purposes for existing drugs. One such ‘old generic’ that has been repurposed and re-registered is thioguanine [1].
Repurposing thioguanine
Generics/Research
|
Posted 06/10/2017
 0
Post your comment
0
Post your comment

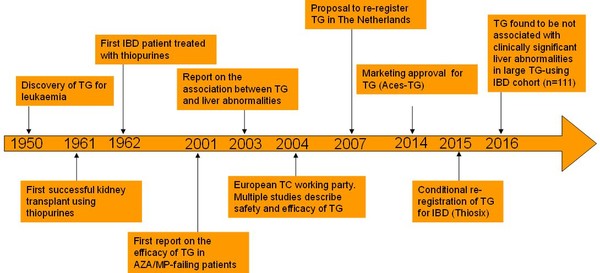
The discovery of the thiopurine derivative, thioguanine, dates back more than 65 years when it was originally developed to treat leukaemia in children, see Figure 1.
Figure 1: Thioguanine timeline from discovery to rediscovery and re‑registration
AZA: azathioprine; IBD: inflammatory bowel disease; MP: mercaptopurine; TG: thioguanine.
Early trials conducted to evaluate the efficacy of thioguanine in inflammatory bowel disease (IBD) treatment described that up to 80% of these patients were able to tolerate this drug and experienced clinical benefit from its use. However, a clinically alarming association of high dosages of thioguanine with the development of nodular regenerative hyper-plasia of the liver overshadowed these findings.
Later trials, however, demonstrated that thioguanine did not increase the risk of liver abnormalities when given in low but therapeutically adequate dosages (0.2−0.3 mg/kg, not exceeding 25 mg per day). In light of these more positive results, a re‑registration of thioguanine was proposed to the Dutch Medicines Evaluation Board (MEB).
This proposal, say the authors, ‘created awareness about drug rediscovery, and the importance of safe and efficient use of existing drugs was recognized by health institutes in The Netherlands’. However, they also point out that ‘there is a need for a structured protocol for further development of old drugs to optimize licensing and avoid long-lasting procedures, as was the case in the repositioning of thioguanine for IBD in The Netherlands’.
Related articles
Challenges in the rediscovery of old generics
Repurposing and re-registering old generics
Reference
1. Simsek M, Meijer B, van Bodegraven AA, de Boer NKH, Mulder CJJ. Finding hidden treasures in old drugs: the challenges and importance of licensing generics. Drug Discov Today. 2017 Sep 1. doi:10.1016/j.drudis.2017.08.008. [Epub ahead of print]
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2017 Pro Pharma Communications International. All Rights Reserved.
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
Japan’s drug shortage crisis: challenges and policy solutions
Saudi FDA drug approvals and GMP inspections: trend analysis
Generic medications in the Lebanese community: understanding and public perception
Community pharmacists’ understanding of generic and biosimilar drugs: Lebanon case study
Generic medications in the Lebanese community: understanding and public perception

Generics/Research Posted 23/01/2024
Community pharmacists’ understanding of generic and biosimilar drugs: Lebanon case study

Generics/Research Posted 08/09/2023
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment