A study carried out in the Czech Republic found that whether pharmacists routinely carried out generics substitution depended on their familiarity with the relevant legislation and their attitude towards generics substitution [1].
Pharmacists’ attitudes towards generics in Czech Republic
Generics/Research
|
Posted 16/05/2014
 0
Post your comment
0
Post your comment

Although generics substitution has long been practised in the Czech Republic, Act No. 378/2007 on pharmaceuticals provides an explicit definition of generics substitution.
In the study by Maly and co-authors pharmacists were questioned on their opinions, attitudes and experiences with generics substitution in the Czech Republic. The study was carried out via a survey among Czech Chamber of Pharmacists (CCP) members from November 2008 to March 2009.
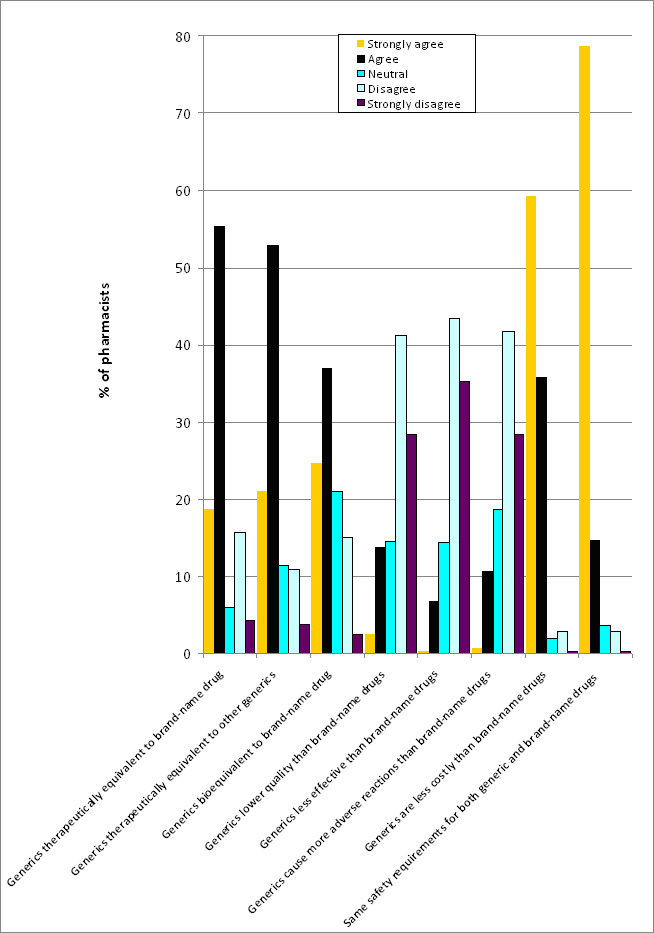
Of the 615 pharmacists that completed the questionnaire 61.5% considered generics as bioequivalent to the respective brand-name drugs and 74% as therapeutically equivalent to the respective brand-name drugs as well as to one another. Most pharmacists (95%) believed that generics were cheaper than brand-name drugs. The majority (93.3%) believed that the law requires the same safety measures and the same production quality guarantee (compliance with good manufacturing practice) for generics as for brand-name drugs. Most pharmacists also considered generics to be comparable to brand-name drugs in terms of quality (69.4%), efficacy (78.7%) and incidence of adverse drug reactions (70.1%), see Figure 1.
Figure 1: Pharmacists attitudes towards generics in the Czech Republic
Generics substitution was viewed as positive by 77.4% of pharmacists, with only 5.2% considering it as negative and none of the respondents rating generics substitution as very negative. A statistically significant correlation was found between understanding the legislation for generics substitution and pharmacist’s attitude towards generics substitution. Younger pharmacists were also found to have a better knowledge of generics substitution and a more positive attitude towards generics substitution.
The authors concluded that generics substitution in Czech Republic seems to be closely associated with the pharmacist’s confidence in generics and generics substitution. This confidence depends, among other things, on pharmacists understanding of the legislation for generics substitution.
Conflict of interest
The authors of the research paper [1] did not report any conflicts of interest.
Editor’s comment
Readers interested to learn more about generics substitution are invited to visit www.gabi-journal.net to view the following manuscripts published in GaBI Journal:
Generics substitution in primary care: summary of the Dutch community pharmacies guidelines
If you are interested in contributing a research paper in a similar area to GaBI Journal, please send us your submission here.
Related articles
Attitudes towards generics in Turkey
US pharmacists positive about generic drugs
Reference
1. Maly J, Dosedel M, Kubena A, Vlcek J. Analysis of pharmacists' opinions, attitudes and experiences with generic drugs and generic substitution in the Czech Republic. Acta Pol Pharm. 2013;70(5):923-31.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2014 Pro Pharma Communications International. All Rights Reserved.
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
Japan’s drug shortage crisis: challenges and policy solutions
Saudi FDA drug approvals and GMP inspections: trend analysis
Generic medications in the Lebanese community: understanding and public perception
Community pharmacists’ understanding of generic and biosimilar drugs: Lebanon case study
Generic medications in the Lebanese community: understanding and public perception

Generics/Research Posted 23/01/2024
Community pharmacists’ understanding of generic and biosimilar drugs: Lebanon case study

Generics/Research Posted 08/09/2023
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment