A generic formulation of atorvastatin was developed in Korea and approved by Korea Food and Drug Administration on 1 July 2008. This clinical trial was conducted at ten clinical centres in Korea between September 2008 and May 2010 for the purposes of marketing the generic formulation [1].
Efficacy and tolerability of generic and brand-name atorvastatin
Generics/Research
|
Posted 01/06/2012
 0
Post your comment
0
Post your comment
Background: The reduction in plasma low-density-lipoprotein cholesterol (LDL-C) concentrations with 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor (statin) therapy has been reported to reduce cardiovascular risk and mortality in individuals with or without pre-existing coronary artery disease and elevated LDL-C concentrations. Atorvastatin is a statin used for lowering LDL-C concentrations.
Objective: This study was designed to compare the efficacy and tolerability of a generic formulation of atorvastatin 20 mg/dose versus a brand-name formulation at the same dosage in hypercholesterolemic Korean adults at high risk of cardiovascular events.
Methods: This multicentre, randomised, double-blind, double-dummy study was conducted over 8 weeks. Male and female patients aged 20 to 85 years at high risk of cardiovascular events (defined as an elevated LDL-C concentration [≥ 100 mg/dL]) were enrolled. Eligible patients were randomly assigned to receive generic or brand-name atorvastatin 20 mg once a day. The primary endpoint was the percentage change from baseline to 8 weeks in LDL-C concentration. Tolerability was assessed using physical examination, laboratory testing, and by recording adverse events (AEs) at each visit. A number of secondary endpoints were also assessed.
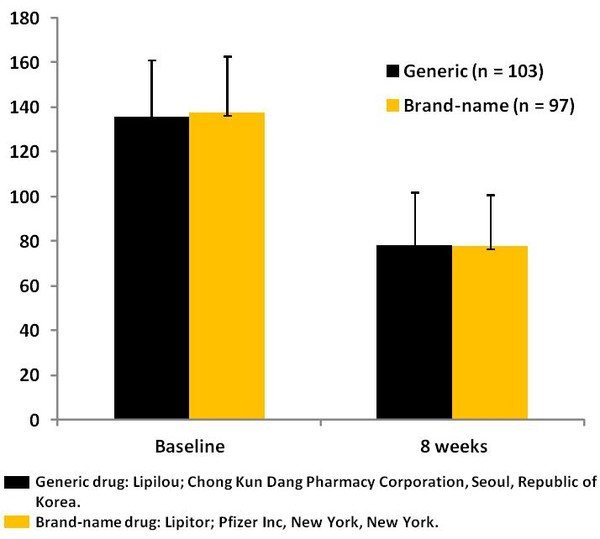
Results: A total of 244 patients were randomised to treatment and 33 patients were withdrawn from the study (nine patients did not receive the study medicine, 11 patients due to AEs and 13 patients due to withdrawal of consent). A total of 211 patients completed the study (50.7% male; 100% Asian; mean [SD] age, 61.7 [9.2] years) (106 patients in the group that received the generic formulation and 105 patients in the group that received the brand-name formulation). LDL-C concentrations were reduced from baseline by 44% and 46% after 8 weeks of treatment with the generic and branded formulations, respectively. There were no significant differences between the two groups, see Figure 1.
Figure 1: Mean (SD) LDL-C concentrations at baseline and after 8 weeks of treatment with generic or branded formulations of atorvastatin 20 mg/dose in Korean patients with hypercholesterolemia [1]
None of the secondary endpoints achieved a statistically significant change within the 8 weeks of the study.
The differences in the overall frequency of AEs were not statistically significant between the two treatment groups. Adverse events were reported in 27.7% (33/119) of patients who received the generic formulation and 31.0% (36/116) of patients who received the brand-name formulation. Adverse events considered unrelated to the study medicine were reported in 21.8% (26/119) of patients in the generic drug group and 29.3% (34/116) of patients in the brand-name group; the three most common AEs were common cold (6.4%), gastrointestinal abnormalities (6.0%) and musculoskeletal discomfort (4.7%). No serious AEs were reported in either group.
The rates of achieving the LDL-C goal at week 8 were not significantly different between the generic drug and brand-name groups.
Discussion: The study had several limitations. It enrolled only a small number of patients. Drug compliance was measured using a pill count, which is not considered an accurate measure of compliance. The duration of follow-up might be considered too brief to effectively compare the efficacy and tolerability of these two formulations of atorvastatin.
Conclusion: After 8 weeks of treatment, there was no statistical difference between the LDL-C-lowering effects of the generic and brand-name formulations of atorvastatin 20 mg. In other words, both formulations appeared equally effective and well tolerated.
Conflict of interest
This research was financially supported by Chong Kun Dang Pharmacy Corporation, Seoul, Republic of Korea. The funding body had no role in the analysis and interpretation of the data. The authors have indicated that they have no other conflicts of interest with regard to the content of this article.
Editor’s comment
If you are interested in contributing a research article in a similar area to GaBI Journal, please send us your submission here.
Related articles
Can generic competition succeed at reducing cost of atorvastatin?
Increased use of generic statins proves cost effective in Australia
Bioavailability comparison of brand-name and generic acetylcysteine in China
Reference
1. Kim SH, Park K, Hong SJ, Cho YS, Sung JD, Moon GW,et al. Efficacy and tolerability of a generic and a branded formulation of atorvastatin 20 mg/d in hypercholesterolemic Korean adults at high risk for cardiovascular disease: a multicenter, prospective, randomized, double-blind, double-dummy clinical trial. Clin Ther. 2010 Oct;32(11):1896-905.
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
Japan’s drug shortage crisis: challenges and policy solutions
Saudi FDA drug approvals and GMP inspections: trend analysis
Generic medications in the Lebanese community: understanding and public perception
Community pharmacists’ understanding of generic and biosimilar drugs: Lebanon case study
Generic medications in the Lebanese community: understanding and public perception

Generics/Research Posted 23/01/2024
Community pharmacists’ understanding of generic and biosimilar drugs: Lebanon case study

Generics/Research Posted 08/09/2023
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment