Hepatitis is an inflammation of the liver and can be caused by medications, toxins, severe alcohol use and other medical conditions. Hepatitis can also be caused by viruses, most commonly hepatitis A, B and C. Both hepatitis A and B can be dangerous, but there are effective vaccines against both viruses, however, there is no vaccines for hepatitis C [1].
Effectiveness of chronic hepatitis C treatment using direct-acting antivirals: real-life data
Generics/Research
|
Posted 30/11/2018
 0
Post your comment
0
Post your comment

Viral hepatitis C causes both acute and chronic infections. Commonly acute hepatitis C is asymptomatic and is rarely associated with life-threatening conditions. In approximately 15‒45% of the cases the virus spontaneously disappears within six months without any treatment. However, in the remaining 60‒80% of cases chronic hepatitis C infection will develop and the risk of developing cirrhosis of the liver within 20 years is 15‒30% [2].
According to an epidemiological study carried out in 2008, there were more than 39,000 patients with chronic hepatitis C in Latvia [3].
Since January 2016, the direct-acting antiviral (DAA) medicines ombitasvir, paritaprevir/ritonavir and dasabuvir have been 100% reimbursed in Latvia. Elbasvir, grazoprevir was included into the reimbursement list from 1 January 2017 and since 1 February 2018 patients with all genotypes are eligible for reimbursed treatment. As of 1 February 2018, there is only one combined DAA medicine reimbursed – sofosbuvir/velpatasvir [4].
The remaining group of chronic hepatitis C patients who have no access to state reimbursed treatment with newer medicines, e.g. those with fibrosis stage F0, F1 and F2, either stay without treatment or choose to obtain generics directly from India.
A retrospective study was performed in Riga East University Hospital, Latvian Centre of Infectious Diseases. Data was obtained from patients’ medical records in the period from November 2016 to March 2017 [5]. Patients were divided into five groups. Each group presents data on a specific medication scheme, using the HCV RNA after treatment at Week 12*. The aim of the study was to evaluate the effectiveness and benefits of DAA medication treatment in Latvia.
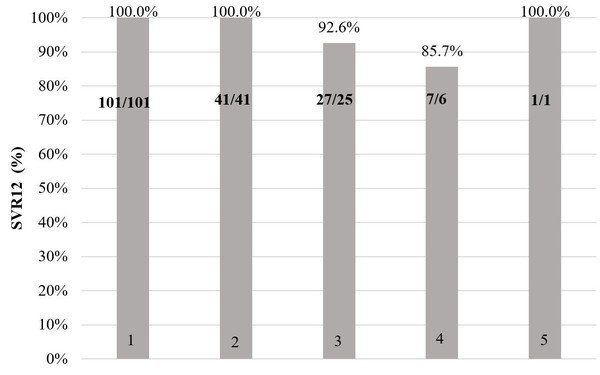
Figure 1 shows sustained virological response (meaning that the hepatitis C virus is not detected in the blood) results 12 weeks after treatment completion for patients who completed treatment (2 patients in the original DAA group discontinued treatment due to decompensation of liver cirrhosis or side effects).
Figure 1:Sustained virological response after treatment completion
1 – Ombitasvir, paritaprevir/ritonavir, dasabuvir
2 – Ombitasvir, paritaprevir/ritonavir, dasabuvir + ribavirin
3 – Sofosbuvir, daclatasvir
4 – Sofosbuvir, ledispavir
5 – Sofosbuvir, daclatasvir + ribavirin
In the 101 patients that received ombitasvir, paritaprevir/ritonavir, dasabuvir HCV RNA 12 weeks after treatment was not detected in 100% (n = 101). This was also true for the 41 patients that received ombitasvir, paritaprevir/ritonavir, dasabuvir + ribavirin [100% (n = 41)]. In the 27 patients that received daclatasvir, sofosbuvir 92.6% (n = 25) had no detectable HCV RNA 12 weeks after treatment. For this treatment regime, the therapy was ineffective in 3.7% (n = 1) and relapsed in 3.7% (n = 1) of cases. In the 7 patients that received ledispavir, sofosbuvir 85.7% (n = 6) had no detectable HCV RNA 12 weeks after treatment and 14.3% (n = 1) relapsed. For the one patient that received daclatasvir, sofosbuvir + ribavirin HCV RNA 12 weeks after treatment was not detected 100% (n = 1).
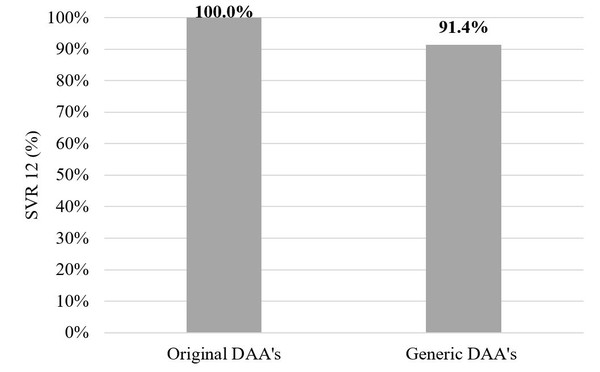
Of those who completed the treatment course in the original DAA group – 142 patients – all 142 (100%) patients achieved a sustained virological response at Week 12 (SVR12). But in the generic DAA group SVR12 was achieved in 32 patients (91.4%, p = 0.007, Fischer’s test). In the original DAA group two patients (1.4%) stopped therapy earlier due to decompensation of liver cirrhosis or side effects. In the generic DAA group SVR12 was not achieved in 3 patients (8.6%) – 1 patient was HCV-RNA positive immediately after treatment and 2 had relapses.
Figure 2 shows the comparison between originator DAAs and generics.
Figure 2: Originator versus generic DAAs
DAA: direct-acting antiviral.
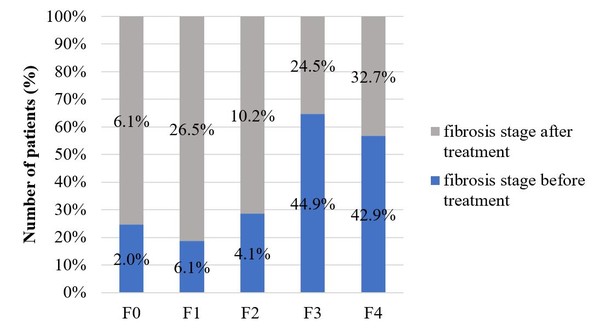
All included patients underwent liver biopsy for fibrosis staging before treatment. Only 49 patients had a FibroScan examination after biopsy. Before the start of therapy there were several patients with cirrhosis and third stage fibrosis, but after the completion of treatment the occurrence of fibrosis stage 1 (before treatment 6.1%, after treatment 26.5%) and fibrosis stage 2 increased (before treatment 4.1%, after treatment 10.2%). This indicates that by reaching an SVR liver tissues are able to regenerate. Figure 3 shows the fibrosis stages before and after treatment in those patients who had a FibroScanexamination after treatment completion.
Figure 3: Fibrosis stages before and after treatment
The results show that all treatment regimes are effective. The effectiveness of state compensated medicines and generics was found to be equal (p = 0.007 Fisher’s direct test). The authors therefore concluded that the use of generics is well-founded and should be recommended for patients.
*Undetectable or unquantifiable HCV RNA 12 weeks or longer after treatment completion is defined asanSVR,which is consistent with cure of hepatitis C infection.
Conflict of interest
The authors of the research paper [5] declared that there was no conflict of interest.
Related article
Generic sofosbuvir and daclatasvir bioequivalent to originators
References
1. Centers for disease Control and Prevention. Hepatitis A questions and answers for the public. 2018 [homepage on the Internet]. [cited 2018 Nov 30]. Available from: www.cdc.gov/hepatitis/hav/afaq.htm
2. World Health Organization. Hepatitis C. 2018 [homepage on the Internet]. [cited 2018 Nov 30]. Available from: www.who.int/news-room/fact-sheets/detail/hepatitis-c
3. Tolmane I, Rozentāle B, Keišs J, et al. The prevalence of viral hepatitis C in Latvia: a population-based study. Medicina (Kaunas). 2011;47(10):532-5.
4. Nacionālais veselības dienests (NVD) [The National Health Service (NHS)]. Kompensējamo zāļu saraksts [Current reimbursement criteria data] [homepage on the Internet]. [cited 2018 Nov 30]. Available from: www.vmnvd.gov.lv/lv/kompensejamie-medikamenti/kompensejamo-zalu-saraksts
5. Tolmane I, Rozentale B, Arutjunana S, et al. Original versus generic direct acting antivirals in treatment of chronic hepatitis C patients: real life data from Latvia. Open Infect Dis J. 2018;10(1):63-70.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2018 Pro Pharma Communications International. All Rights Reserved.
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
Japan’s drug shortage crisis: challenges and policy solutions
Saudi FDA drug approvals and GMP inspections: trend analysis
Generic medications in the Lebanese community: understanding and public perception
Community pharmacists’ understanding of generic and biosimilar drugs: Lebanon case study
Generic medications in the Lebanese community: understanding and public perception

Generics/Research Posted 23/01/2024
Community pharmacists’ understanding of generic and biosimilar drugs: Lebanon case study

Generics/Research Posted 08/09/2023
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment