In their review, Machado et al. examine the transparency and regulatory guidelines pertaining to the licensing of biosimilars, as well as the count of biosimilars granted approval by 13 medicines regulatory authorities [1].
First approvals of similar biotherapeutics in seven Latin American countries
Home/Reports
|
Posted 16/01/2024
 0
Post your comment
0
Post your comment
This article discusses the first biosimilar to receive approval following the issuance of licensing guidelines by regulatory authorities around the world.
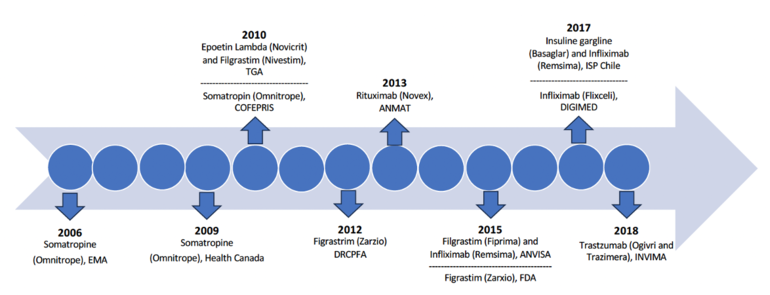
In 2006, the European Medicines Agency became the pioneer regulatory authority to authorize a biosimilar – Sandoz's human growth hormone somatropin, a reference to Omnitrope. Health Canada and Federal Commission for the Protection against Health Risks (Comisión Federal para la Protección contra Riesgos Sanitarios, COFEPRIS) followed suit in 2009 and 2010, respectively, endorsing the same product, albeit referred to as biocomparable in Mexico at the time. Subsequent approvals for the first biosimilars by Australia's Therapeutic Goods Administration (TGA) and the US Food and Drug Administraion (FDA) occurred in 2010 and 2015, as depicted in Figure 1.
ANMAT: Administración Nacional de Medicamentos, Alimentos y Tecnología Médica; ANVISA: Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária); COFEPRIS: Federal Commission for the Protection against Health Risks (Comisión Federal para la Protección contra Riesgos Sanitarios); DIGEMID: Dirección General de Medicamentos, Insumos y Drogas; DRCPFA: Departamento de Regulación y Control de Productos Farmacéuticos y Afines; EMA: European Medicines Agency; FDA: US Food and Drug Administration; INVIMA: Instituto Nacional de Vigilancia de Medicamentos y Alimentos; ISP: Instituto de Salud Pública de Chile; TGA: Therapeutic Goods Administration.
Table 1 presents data of the first follow-on biologicals approved in seven Latin American countries
| Table 1: First follow-on biological approved following the release of licensing guidelines in Latin America | |||
| Year | Regulatory authority (country) |
Nonproprietary name (brand name) |
Manufacturer |
| 2010 | COFEPRIS (Mexico) | Somatropin (Omnitrope) | Sandoz |
| 2012 | DRCPFA (Guatemala) | Filgrastim (Zarzio) | Sandoz |
| 2013 | ANMAT (Argentina) | Rituximab (Novex) | Elea Phoenix |
| 2015 | ANVISA (Brazil) |
Filgrastim (Fiprima) Infliximab (Remsina) |
Eurofarma Laboratório Celltrion |
| 2017 | ISP (Chile) |
Infliximab (Remsina) Insulin gargline (Basaglar) |
Laboratorios Saval Eli Lilly |
| 2017 | DIGEMID (Peru) | Infliximab (Flixceli) | Celltrion |
| 2018 | INVIMA (Colombia) |
Trastuzumab (Ogivri) Trastuzumab (Trazimera) |
Mylan GmbH Pfizer |
| ANMAT: Administración Nacional de Medicamentos, Alimentos y Tecnología Médica; ANVISA: Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária); COFEPRIS: Federal Commission for the Protection against Health Risks (Comisión Federal para la Protección contra Riesgos Sanitarios); DIGEMID: Dirección General de Medicamentos, Insumos y Drogas; DRCPFA: Departamento de Regulación y Control de Productos Farmacéuticos y Afines; INVIMA: Instituto Nacional de Vigilancia de Medicamentos y Alimentos. |
Since then, additional follow-on biologicals have been approved by the respective regulatory authorities in Latin America, notably Brazil’s ANVISA despite Mexico’s COFEPRIS is the first regulatory authority to have approved a biocomparable.
By mid-May of 2023, ANVISA had approved 52 follow-on biological/biosimilar products within the classes of: 1) human growth hormone; 2) granulocyte colony-stimulating factor; 3) insulin; 4) tumour necrosis factor (TNF)-inhibitor; and 5) monoclonal antibody for use in Brazil [2].
Investment in biosimilars in Latin American countries is increasing year on year and regulatory authorities in many Latin American countries have been redesigned and standardized for the approval of biosimilars. However, local regulations vary between countries [3].
Related articles
Study of the use of generic and biosimilar drugs in Latin America
Recommendations to address challenges to biosimilars in Latin America
Similar biotherapeutic products approved and marketed in Latin America
|
LATIN AMERICAN FORUM View the latest headline article: Consulta pública para la modificación de la regulación de biosimilares Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: Consulta pública para la modificación de la regulación de biosimilares !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. Machado FLDS, Cañás M, Doubova SV, Urtasun MA, Marín GH, Osorio-de-Castro CGS, et al. Biosimilars approvals by thirteen regulatory authorities: A cross-national comparison. Regul Toxicol Pharmacol. 2023 Sep 1;144:105485. doi: 10.1016/j.yrtph.2023.105485. 2.
2. Cestari de Oliveira SH. Follow-on biologicals/biosimilars approved in Brazil: May 2023 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2023;12(2):67-72. doi:10.5639/gabij.2023.1202.012
3. Ortiz-Prado E, Ponce-Zea J, Vasconez JE, et al. Current trends for biosimilars in the Latin American market. Generics Biosimilars Initiative Journal. (GaBI Journal). 2020;9(2):64-74. doi:10.5639/gabij.2020.0902.011
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2024 Pro Pharma Communications International. All Rights Reserved.
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment