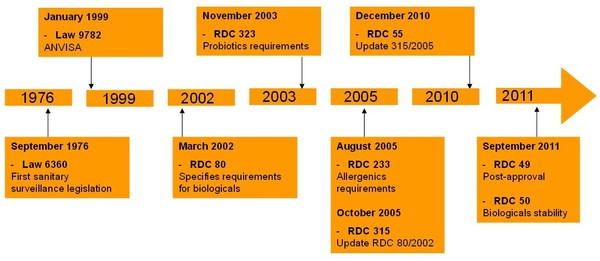
The regulatory body for approval of medicines in Brazil is the Brazilian Health Surveillance Agency (Agência Nacional de Vigilância Sanitária, ANVISA) [1]. The agency was created by Law 9782, which was enacted in 1999 [2]. Since then, numerous guidance for biologicals and follow-on biological products have been issued by ANVISA, see Figure 1.
Up until 2002, Brazil had no specific guidance for biological products. In 2002, guidelines for biological products were published (RDC 80/2002), which had to be followed by both originator biologicals and ‘follow-on biological products’. In 2005, these regulations were revised, however, no alternate pathway for follow-on biological products was specified at the time and a full dossier was still required.
In 2010, Brazil introduced regulations to specifically address and establish specific pathways to license follow-on biological products using a comparability pathway. The Brazilian regulations (RDC 55/2010) are based on different regulations and guidelines from around the world, including the WHO Similar Biological Product Guidelines. They follow the same scientific principles as the WHO guidelines but contain some differences due to the specific needs of Brazil.
Figure 1: Evolution of biologicals regulation in Brazil
Follow-on biologicals approved in Brazil
To date two follow-on biologicals have been approved in Brazil [3].
Remsima
Hospira’s Remsima (infliximab) is a follow-on biological of Johnson & Johnson and Merck’s Remicade (infliximab). It was the first follow-on biological approved by ANVISA in April 2015 [4].
Remsima was granted full label approval, with extrapolation to all indications listed in the package insert of the originator product Remicade.
The price for Remsima (approved in May 2016) is 35% lower than the originator, more than a year after its approval.
Fiprima
Eurofarma Laboratórios’ (Eurofarma) Fiprima (filgrastim) is a follow-on biological of Amgen’s Neupogen (filgrastim). It was the second follow-on biological to be approved by ANVISA in October 2015 [5].
It is the first follow-on biological developed and fully manufactured in Brazil.
The price is expected to be equivalent to the originator product Neupogen. Approval of the price was in May 2016, giving a seven-month delay after approval.
Editor’s comment
It should be noted that ‘follow-on biological products’ approved in Brazil might not have been authorized following as strict a regulatory process as is required for approval of biosimilars in the European Union. The European Medicines Agency (EMA) regulatory requirements ensure the same high standards of quality, safety and efficacy for biosimilars as for originator biologicals, and also include a rigorous comparability exercise with the reference product.
Related articles
Regulatory pathways for approval of biological products in Brazil
Producing follow-on biological products in Brazil
Brazil speeds up approval process for generics and biologicals
References
1. GaBI Online - Generics and Biosimilars Initiative. Brazilian guidelines for follow-on biological products [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Sep 8]. Available from: www.gabionline.net/Guidelines/Brazilian-guidelines-for-follow-on-biological-products
2. Gomez Valente B. Evolution of Biosimilars Regulations in Brazil. 13th European Generic Medicines Association (EGA)-European Biosimilars Group Conference, London, UK 23-24 April 2015.
3. GaBI Online - Generics and Biosimilars Initiative. Similar biotherapeutic products approved and marketed in Latin America [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Sep 8]. Available from: www.gabionline.net/Biosimilars/General/Similar-biotherapeutic-products-approved-and-marketed-in-Latin-America
4. GaBI Online - Generics and Biosimilars Initiative. Brazil approves first monoclonal antibody follow-on biological [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Sep 8]. Available from: www.gabionline.net/Biosimilars/News/Brazil-approves-first-monoclonal-antibody-follow-on-biological
5. GaBI Online - Generics and Biosimilars Initiative. Filgrastim follow-on biological approved in Brazil [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Sep 8]. Available from: www.gabionline.net/Biosimilars/News/Filgrastim-follow-on-biological-approved-in-Brazil
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2017 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment