Professor Tibor Hlavatý, from United European Gastroenterology, which is a European umbrella organisation for gastroenterologists, gave a presentation on the current position of biosimilars in the field of gastroenterology at the fourth European Commission Stakeholder Conference on Biosimilar Medicines, which was held on 14 September 2018 in Brussels, Belgium [1].
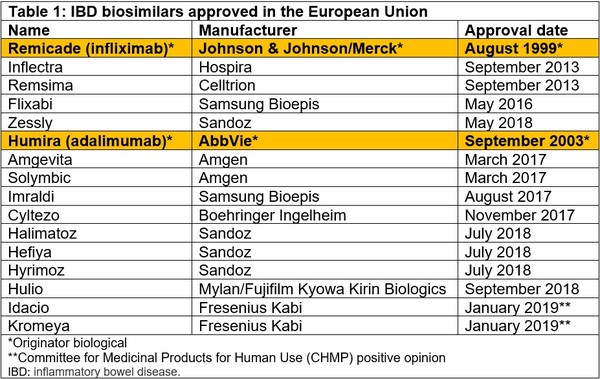
Biosimilars for the treatment of inflammatory bowel disease (IBD) have been available since Inflectra and Remsima (CT-P13) were approved in Europe in September 2013 [2] and Inflectra (infliximab-dyyb) was approved in the US in April 2016 [3]. As of February 2019, there are now four infliximab biosimilars and 10 adalimumab biosimilars approved in Europe, see Table 1.
CT-P13 was the first monoclonal antibody biosimilar approved by both the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA). It was proven to be equivalent to treatment with the originator infliximab, Remicade, in ankylosing spondylitis (PLANETAS) and rheumatoid arthritis (PLANETRA) [4], but there was limited experience with its use in IBD and this caused much discussion in the beginning. During his presentation, Professor Tibor Hlavatý outlined how infliximab treatment has shown significant success in patients with IBD. However, high costs have limited its affordability in many countries.
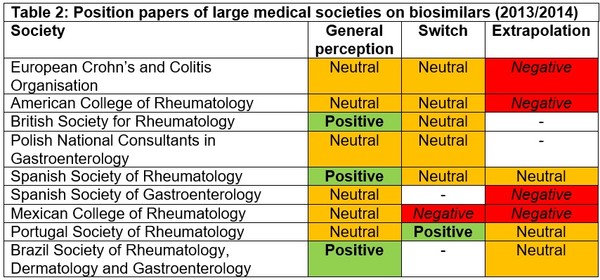
In an effort to come to a consensus and to reassure gastroenterologists when using biosimilars, many large medical societies produced position papers on the subject of biosimilars. These position papers also included opinions on the merits of extrapolation of indications and as to whether their members should switch patients already on stable therapy with the originator biological onto a biosimilar, see Table 2.
The European Crohn’s and Colitis Organisation (ECCO) was initially wary about biosimilars, saying that specific evidence is required for the use of biosimilars in IBD patients [7]. However, when it published results of a consensus meeting held on15 October 2016, they said that they now supported switching from reference infliximab to biosimilar infliximab, as well as extrapolation of indications for biosimilars [8]. However, since these first position papers were published back in 2013/2014, many studies in IBD have been published supporting the biosimilarity of infliximab and adalimumab with their reference products [5]. In light of this, medical societies have updated their position statements on the use of biosimilars for IBD. In fact, the Spanish Society of Gastroenterology (Sociedad Española de Patología Digestiva, SEPD) updated its position paper in 2018 saying that ‘based on the data published, the biosimilar CT-P13 is safe and effective in IBD, both in naïve and switched patients’ [6].
A previous ECCO survey from 2013 had also highlighted a lack of confidence in biosimilars and the need for continued education [8]. Whereas a more recent survey, carried out in 2015 by ECCO pointed to a change in attitudes. Results even showed that only 19.5% felt little or not confident in the use of biosimilars, compared to 63% in 2013. The percentage of prescribers that thought that biosimilars could work differently to the originator biological had reduced from 43.1% to 16.9%. In addition, those that thought that biosimilars could have a different immunogenicity pattern compared to the originator biological had reduced to 27.1% from 67.1% in 2013.
The 2016 ECCO position statement on biosimilars therefore reflects these changing attitudes, saying that:
• Data for usage of biosimilars can be extrapolated from another sensitive indication.
• Need for registries and large observational studies with long-term follow up to demonstrate the safety of biosimilars in IBD.
• Adverse events and loss of response due to immunogenicity to a biological cannot be expected to be overcome with a biosimilar of the same molecule.
• Switching from originator to biosimilar in IBD is acceptable; it should be performed following appropriate discussion between physicians, nurses, pharmacists and patients.
Related articles
Biosimilars in the treatment of inflammatory bowel disease
Hungarian experience with use of biosimilar infliximab in IBD
References
1. Hlavatý T. Current position of biosimilars in the field of gastroenterology (IBD) 2018. Fourth European Commission Stakeholder Conference on Biosimilar Medicines. 14 September 2018; Brussels, Belgium.
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Mar 22]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
3. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Mar 22]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-the-US
4. GaBI Journal Editor. Switching from the infliximab reference product to CT-P13 in patients with rheumatoid arthritis or ankylosing spondylitis: results of the PLANETAS and PLANETRA extension studies Generics and Biosimilars Initiative Journal (GaBI Journal). 2016;5(2):94-5.
5. GaBI Online - Generics and Biosimilars Initiative. Inflectra shows comparable results in IBD patients [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Mar 22]. Available from: www.gabionline.net/Biosimilars/Research/Inflectra-shows-comparable-results-in-IBD-patients
6. GaBI Online - Generics and Biosimilars Initiative. Spanish gastroenterologists update biosimilar position statement [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Mar 22]. Available from: www.gabionline.net/Biosimilars/General/Spanish-gastroenterologists-update-biosimilar-position-statement
7. GaBI Online - Generics and Biosimilars Initiative. ECCO position statement on biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Mar 22]. Available from: www.gabionline.net/Biosimilars/General/ECCO-position-statement-on-biosimilars
8. GaBI Online - Generics and Biosimilars Initiative. European IBD specialists support switching to biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Mar 22]. Available from: www.gabionline.net/Biosimilars/General/European-IBD-specialists-support-switching-to-biosimilars
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2019 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment