Drug shortages in the US are at a record high and there appears to be no quick solution to the problem. Legislation, government stock-piling and physicians importing or even manufacturing drugs are just some of the solutions being proposed.
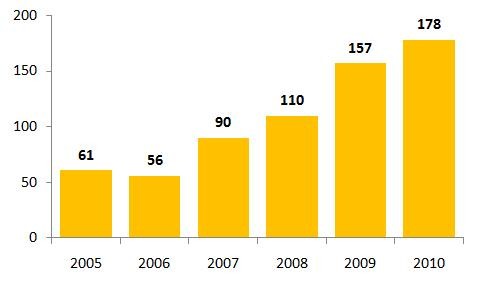
In 2010, there were 178 drugs that experienced shortages in the US, see Figure 1. This is a record high level and, while regulators blame quality and manufacturing problems for the crisis, others blame the FDA’s own policies for causing the problems. Worse still, many of the drugs identified in 2010 remain unavailable or in short supply in 2011 [1]. A report from The Times suggests that so far in 2011, at least 180 drugs that are crucial for treating childhood leukaemia, breast and colon cancer, infections and other diseases are in short supply.
Figure 1: Drug shortages in the US 2005–2010
Source: FDA
Some of FDA’s policies, such as its good manufacturing practice (GMP) rules, have been blamed for clogging up the drug production process and raising costs. Problems with complying with GMP rules are seen as one of the major causes of drug shortages as a result of stoppages in production or recalls. The agency’s Unapproved Drugs Initiative has also been accused of causing manufacturers to withdraw old drugs (those that pre-date the Food, Drug and Cosmetic Act of 1938) from the market, due to the cost and complexity of filing a new drug application for these unapproved drugs [2].
Shortages in supply can cause many problems, not least of which is the fact that the price of essential and often emergency room medications are often inflated. Hospitals report that 45% of drugs in short supply have been offered for sale at more than 10 times the normal rate, while 27% were at least 20 times the normal rate.
In order to prevent such shortages in the future the US Government is considering creating a government stockpile of essential cancer medicines in much the same way as the Centers for Disease Control and Prevention already stockpile vaccines, for example, in case of national emergencies.
Legislation has also been proposed that would demand that drugmakers inform FDA of any impending shortages. At present, manufacturers are not obliged to warn FDA about any expected problems with supply of drugs. This makes it difficult for FDA to prevent drug shortages by working with other firms who manufacturer the drug and asking them to increase production.
In a desperate bid to prevent such frustrating shortages of critical cancer medications, a group of leading oncologists has started a non-profit drug company with the intention of initially importing and eventually manufacturing some of the medicines in short supply.
A lack of FDA inspectors has also been blamed for the problem. President of generic drug giant Mylan, Ms Heather Bresch, is advocating generic drug user fees in order to finance overseas plant inspections. ‘If the FDA was required to inspect foreign drug plants at the same rate it does domestic ones, we might not have so many of these shortages.’ she says.
Whatever the solution generics have a definite beneficial role to play here. Maintaining supply of older injectable drugs and ramping up production when manufacturing problems arise elsewhere are just some of the ways generics can help to prevent such shortages occurring.
Related article
US drug shortages – frustration and safety concerns
References
1. GaBI Online - Generics and Biosimilars Initiative. Record high drug shortages in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2011 September 09]. Available from: www.gabionline.net/Pharma-News/Record-high-drug-shortages-in-the-US
2. GaBI Online - Generics and Biosimilars Initiative. US drug shortages – FDA may hinder, but APP may help [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2011 September 09]. Available from: www.gabionline.net/Generics/News/US-drug-shortages-FDA-may-hinder-but-APP-may-help








 0
0











Post your comment