Last update: 27 November 2020
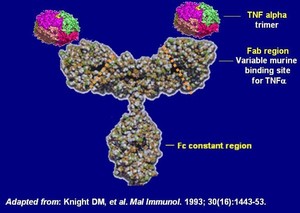
Infliximab is a chimeric monoclonal antibody against tumour necrosis factor alpha (TNF-α). It is used to treat autoimmune diseases, such as ankylosing spondylitis, Crohn’s disease, psoriasis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.
The originator product, Johnson & Johnson and Merck’s Remicade (infliximab), was approved by the US Food and Drug Administration (FDA) in August 1998 and by the European Medicines Agency (EMA) in August 1999 [1]. Remicade had worldwide sales of US$9.3 billion in 2014, before the advent of biosimilars, see Table 1.
The patents on Remicade expired in the US in September 2018 and in Europe in February 2015 [1]. Some of the infliximab biosimilars and non-originator biologicals* approved or in development are presented in Table 1.
| Table 1: Biosimilars and non-originator biologicals* of infliximab approved or in development
|
| Company name, Country
|
Product name
|
Stage of development
|
| Amgen, USA
|
Avsola (ABP 710)
|
Approved by FDA [2] and Health Canada [3] in December 2019 and July 2020, respectively.
|
| Biocad, Russia*
|
BCD-055
|
Non-originator biological approved in Russia in Feb 2018 [4]
|
| BioXpress Therapeutics, Switzerland
|
-
|
Biosimilar in pipeline
|
| Celltrion/Hospira (Pfizer), South Korea/USA
|
Remsima/Inflectra/ Flammegis
(CT-P13)
|
IV version approved in EU in September 2013 [5]. SC version approved by EC in September 2019 [5]. Remsima also has marketing approval in Brazil, Colombia, Japan [6], South Korea [7] and Venezuela. Approved in Canada in January 2014 [8] and in Australia in August 2015 [ 9]. Approved in Russia as Flammegis in July 2015 [4]. Approved by FDA in April 2016 [2]. Received approval to start clinical trials in China from NMPA in May 2017.
|
Ixifi
(PF‑06438179)
|
Pfizer received FDA approval for Ixifi in December 2017 [2]
|
| Epirus Biopharmaceuticals*, USA
|
Infimab
|
‘Similar biologic’ approved in India in September 2014 [10]
|
| MabTech/Sorrento Therapeutics, China*/USA
|
STI-002
|
Positive phase III trial for copy biological in China reported in January 2016
|
| Mabpharm/Sorrento Therapeutics, China*/USA
|
CMAB008
|
Copy biological submitted to China's NMPA for approval in January 2020
|
| Nichi-Iko Pharmaceutical, Japan
|
NI-071
|
Phase III trial in rheumatoid arthritis expected to be completed in March 2015 . Approved in Japan in September 2017 [6]. US phase III trial in rheumatoid arthritis expected to be completed February 2019.
|
| Nippon Kayaku, Japan
|
Infliximab BS
|
Approved in Japan in November 2014 [6]
|
| Ranbaxy Laboratories/Epirus Biopharmaceuticals, India*/USA
|
BOW015
|
‘Similar biologic’ approved in India in December 2014 [10]. Global phase III trial expected to be completed in July 2017.
|
| Samsung Bioepis (Biogen/Samsung)/Merck (MSD), South Korea/USA
|
Flixabi (EU)/Renflexis (US) (SB2)
|
Approved in EU in May 2016 [5]. Approved in Korea as Renflexis in December 2015 [7]. Approved by Australia’s TGA in November 2016 [9]. Approved by FDA in April 2017 [2].
|
| Sandoz, Switzerland
|
Zessly (PF‑06438179)
|
Sandoz acquired EEA rights from Pfizer in February 2016. Approved by EC in May 2018 [5]
|
| Shanghai Biomabs Pharmaceuticals, China*
|
Baimaibo
|
Phase III trial in RA in China started March 2018
|
EC: European Commission; EEA: European Economic Area, this area includes the 28 EU Member States, plus Iceland, Liechtenstein and Norway; EMA: European Medicines Agency; EU: European Union; FDA: US Food and Drug Administration; NMPA: National Medical Products Administration (formerly CFDA: China Food and Drug Administration); RA: rheumatoid arthritis; TGA: Therapeutic Goods Administration.
*See editor’s comment
|
Celltrion/Hospira received approval for the IV version of their infliximab biosimilar (Remsima/Inflectra) in Europe in September 2013 and for the subcutaneous version, Remsima SC, in November 2019 [5]. Inflectra was approved by FDA on 5 April 2016 [2]. Celltrion and Hospira’s infliximab biosimilar (Remsima/Inflectra) has been approved by the EMA for the same indications as Remicade (inflixmab), i.e., ankylosing spondylitis, Crohn’s disease, psoriasis, psoriatic arthritis, rheumatoid arthritis and ulcerative colitis [5]. Remsima will be distributed in Belgium, Germany, Italy, Luxembourg, The Netherlands and the UK exclusively by Mundipharma International and its independent associated companies [12]. Pfizer also received approval for Ixifi (PF‑06438179) from FDA in December 2017 [2]. Amgen’s Avsola (ABP 710) received approval from FDA and Health Canada in December 2019 and July 2020, respectively [2, 3]
Editor’s comment
European Medicines Agency regulatory requirements ensure the same high standards of quality, safety and efficacy for biosimilars as for originator biologicals, and also include a rigorous comparability exercise with the reference product but they are not universally accepted by regulatory bodies outside of the European Union (EU). It should be noted that ‘similar biotherapeutic products’ approved in Latin America, ‘copy biologicals’ approved in China, ‘similar biologics’ approved in India and non-originator biologicals approved in Russia might not have been authorized if they had been subjected to the strict regulatory processes required for approval of biosimilars in the EU.
Related articles
Biosimilars of certolizumab pegol
Biosimilars of adalimumab
Biosimilars of etanercept
References
1. Derbyshire M. Patent expiry dates for biologicals: 2018 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2019;8(1):24-31. doi:10.5639/gabij.2019.0801.003
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Nov 27]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-the-US
3. GaBI Online - Canada approves infliximab and filgrastim biosimilars Avsola and Nivestym [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Nov 27]. Available from www.gabionline.net/Biosimilars/News/Canada-approves-infliximab-and-filgrastim-biosimilars-Avsola-and-Nivestym
4. GaBI Online - Generics and Biosimilars Initiative. Non-originator biologicals approved in Russia [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Nov 27]. Available from:
www.gabionline.net/Biosimilars/General/Non-originator-biologicals-approved-in-Russia
5. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Feb 13]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
6. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Japan [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Nov 29]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Japan
7. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in South Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Nov 27]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-South-Korea
8. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Canada [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Nov 10]. Available from: www.gabionline.net/Biosimilars/General/Subsequent-entry-biologics-approved-in-Canada
9. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Australia [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Nov 10]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Australia
10. GaBI Online - Generics and Biosimilars Initiative. ‘Similar biologics’ approved and marketed in India [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 Nov 10]. Available from: www.gabionline.net/Biosimilars/General/Similar-biologics-approved-and-marketed-in-India
11. GaBI Online - Generics and Biosimilars Initiative. Infliximab biosimilar to be distributed by Mundipharma [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Nov 29]. Available from: www.gabionline.net/Biosimilars/News/Infliximab-biosimilar-to-be-distributed-by-Mundipharma
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2016 Pro Pharma Communications International. All Rights Reserved.
Source: EMA, Johnson & Johnson, Merck








 0
0










Post your comment