Generics/Research
Use of generic anti-asthmatic drugs in Morocco
Asthma is a serious public health problem in Morocco. However, due to low income and lack of healthcare coverage in the country many of Morocco’s citizens do not have access to anti-asthmatic drugs.
Therapeutic substitution could save Americans money
Substituting more expensive brand-name drugs with generics or therapeutic substitutes, could lead to significant savings for Medicare Part D beneficiaries, according to US researchers [1].
Strategies used by sickness funds to increase generics prescribing in Austria
In 2010, a survey of Austrian sickness funds was undertaken to evaluate how Austrian doctors, patients and pharmacists are encouraged to enhance the rational use of medicines, and increase use of generics [1].
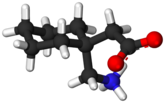
Interchangeability of gabapentin generic formulations in The Netherlands: summary of a comparative bioavailability study
Abstract
The registration of generic formulations in the EU is governed by regulatory authorities in the respective European countries. Before a generic drug can be registered, bioequivalence to the branded formulation has to be demonstrated, i.e. 90% confidence intervals of area under the curve (AUC), and Cmax should fall within the 80–125% acceptance range. Comparative studies with other generics are not required. In order to establish whether a generic medicine is interchangeable with other generic formulations under current regulations, the Dutch regulatory authority conducted a single- dose, four-way crossover comparative bioavailability study with four gabapentin formulations, i.e. the branded formulation and three generic drugs, as test medication. The results showed that all four formulations were bioequivalent to each other, and that no interchangeability issues would result from switching.
How regional sickness funds in Austria encourage more rational prescribing
In Austria, as in other EU countries, rational use of medicines is high on the healthcare agenda. According to WHO, use of generic medicines help reduce costs incurred by public payers and is a way of supporting rational use of medicines.
Implications of the changes in venlafaxine utilization in Sweden following generics
Godman and co-authors review the changes in the utilization of venlafaxine post generics. This includes the limited influence on utilization post generics with no specific demand-side measures. This changed following prescribing restrictions for duloxetine. However, influence limited by the need to tailor treatments for patients with depression [1].
The cost of evergreening strategies
The practice of evergreening, where pharmaceutical companies make small patentable changes to existing products with soon-to-expire patents, contributed to increased healthcare costs in Switzerland, according to a study by authors from the University of Geneva and Geneva University Hospitals [1].
Use of brand-name drugs increasing healthcare costs in US
Patients with diabetes who are covered by the US healthcare insurance Medicare are two to three times more likely to use expensive brand-name drugs compared to patients treated within the Veterans Affairs Healthcare System [1].
FDA grant to study generic versus brand-name transplant drugs
FDA has given a grant to the University of Cincinnati, Ohio, USA, in order to support a study into a commonly used immunosuppressive drug for transplant patients, tacrolimus.
Generic antiepileptic drugs bioequivalent to brand-name drugs
Results of a study, reported by Dr Ravi Juluru and co-authors at the American Epilepsy Society’s 66th Annual Meeting, held in San Diego, USA on 30 November–4 December 2012, shows that generic slow-release drugs for seizure disorders are ‘equivalent’ to their brand-name counterparts [1].