Generics
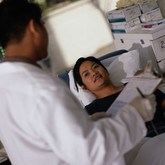
Transplant coordinators’ perception of generic immunosuppressants
Transplant coordinators associate the availability of multiple generic immunosuppression therapies with increased patient confusion, according to a study by Parker and co-authors [1].
Generics applications under review by EMA – 2013 Q3
Last update: 4 October 2013
Generic medicines in Europe can be approved either centrally via the European Medicines Agency (EMA) or nationally via the local regulatory body [1]. Approval by EMA takes place by using a centralized procedure. This leads to approval of the product in all 27 EU Member States and in Norway, Iceland and Liechtenstein. At a country level, if approval in a single EU Member State only is required, this can take place using the national procedure. However, as soon as a company seeks approval in two or more Member States, a decentralized procedure or mutual recognition procedure must be used [2].
Generics of fish oil cholesterol treatment get go ahead
A US appeals court ruled on 12 September 2013 that generics companies can develop their own versions of Lovaza, a fish oil-derived drug used to treat high cholesterol, which is currently marketed in the US by GlaxoSmithKline (GSK).
Australian generics association releases new code of practice
Australia’s Generic Medicines Industry Association (GMiA) announced on 26 September 2013 the release of the draft third edition of the GMiA Code of Practice, which incorporates a number of new amendments.
Advair could face competition from generics as early as 2016
GlaxoSmithKline’s (GSK) asthma and chronic obstructive pulmonary disorder treatment Advair (fluticasone/salmeterol) could face competition from generics sooner than expected, after FDA issued draft guidance on generics of the drug.
Ranbaxy receives another import ban from FDA
Ranbaxy Laboratories (Ranbaxy) has come into FDA’s firing line again. This time generics manufacturer is under scrutiny for problems at its Mohali manufacturing site.
Use of generic anti-asthmatic drugs in Morocco
Asthma is a serious public health problem in Morocco. However, due to low income and lack of healthcare coverage in the country many of Morocco’s citizens do not have access to anti-asthmatic drugs.
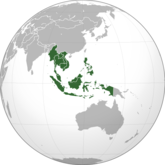
Southeast Asian generics market to reach US$3.9 billion by 2016
Southeast Asia, with its fast-growing, young population and uninsured majority represent a great opportunity for generics in the pharmaceutical industry, according to Rhett Hemedes, Head of OTC Marketing, Great Eastern Drug Co.
Avanir and Wockhardt settle Nuedexta patent dispute
US-based Avanir Pharmaceuticals (Avanir) announced on 6 September 2013 that it had entered into a settlement agreement with Wockhardt USA, the US subsidiary of India-based generics maker Wockhardt, regarding Nuedexta (dextromethorphan hydrobromide/quinidine sulfate).
Actavis confirms Rayos patent challenge and Warner Chilcott acquisition
Actavis has filed for US approval for a generic version of the anti-inflammatory or immunosuppressive agent Rayos (prednisone). The company also confirmed its proposed acquisition of Warner Chilcott.