Biosimilars of Roche’s Avastin (bevacizumab), a humanized monoclonal antibody, are currently under development.
Pivotal clinical trials for bevacizumab biosimilars
Home/Reports
|
Posted 18/09/2015
 0
Post your comment
0
Post your comment
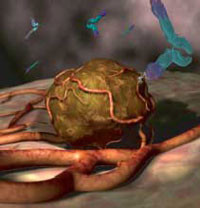
Bevacizumab inhibits angiogenesis (the formation of new blood vessels) by blocking the action of vascular endothelial growth factor A (VEGF-A). Bevacizumab can therefore slow the growth of new blood vessels in tumours and is used to treat various cancers, including colorectal, lung, breast, glioblastoma, kidney and ovarian.
Pivotal trials for candidate bevacizumab biosimilars being carried out typically include just one study of the response rate with some progression-free survival data. The trials take 24–48 months and are tougher trials to recruit and execute than anti-TNF trials [1], see Table 1.
Table 1: Pivotal clinical trials for candidate bevacizumab biosimilars
| Study ID | Product name | Company name, country | Phase | Trial design | Status | No. of subjects | Start/end dates | Primary outcome(s) |
| Lung cancer | ||||||||
| NCT01966003 | ABP 215 | Amgen, USA | III | 2-arm, parallel | Ongoing | 642 | Oct 2013/ Sep 2015 | ORR |
| NCT01763645 | BCD-021 | Biocad, Russia | III | 2-arm, parallel | Ongoing | 214 | Oct 2012/Nov 2015 | ORR, AUC |
| NCT02272413 | BI 695502 | Boehringer Ingelheim, Germany | III | 2-arm, parallel | Recruiting | 660 | Jul 2015/Mar 2019 | ORR |
| 2014-003878-16 | PF-06439535 | Pfizer, USA | III | 2-arm, parallel | Ongoing | 798 | Feb 2015/- | ORR |
| AUC: area under the curve; ORR: overall response rate. | ||||||||
The originator product, Roche’s Avastin (bevacizumab), was approved by the US Food and Drug Administration in February 2004 and by the European Medicines Agency in January 2005. The patents on Avastin are set to expire in Europe in January 2022 and in the US in July 2019 [2]. Avastin had worldwide sales of US$7.4 billion in 2014, making it a lucrative target for biosimilars manufacturers.
Related articles
Pivotal clinical trials for trastuzumab biosimilars
Pivotal clinical trials for rituximab biosimilars
Clinical programs for oncology biosimilars
References
1. Gal R. Biosimilars: at the inflection point. 13th EGA-European Biosimilars Group Conference; 23–24 April 2015; London, UK.
2. GaBI Online - Generics and Biosimilars Initiative. US$67 billion worth of biosimilar patents expiring before 2020 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Sep 18]. Available from: www.gabionline.net/Biosimilars/General/US-67-billion-worth-of-biosimilar-patents-expiring-before-2020
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2015 Pro Pharma Communications International. All Rights Reserved.
Guidelines
New decree for the prescription and commercialization of medicines in Argentina
FDA issues draft guidance on biosimilars and interchangeable biosimilars labelling
Policies & Legislation
Strategic plans of ANVISA and COFEPRIS to advance health regulation
Panama enacts new bill to guarantees the supply of medicines
Follow-on biological/biosimilar approvals in Latin America by therapeutic class

Home/Reports Posted 03/04/2024
First approvals of similar biotherapeutics in seven Latin American countries
Home/Reports Posted 16/01/2024
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment