ABP 980 (Kanjinti) is a biosimilar to trastuzumab reference product (RP) (Herceptin), a monoclonal antibody directed against human epidermal growth factor receptor 2 (HER2). A biosimilar is highly similar to a licensed biological with no clinically meaningful differences in safety, purity and potency [1, 2]. Kanjinti is approved in the US, European Union, and as trastuzumab BS [trastuzumab biosimilar 2] in Japan for all Herceptin indications, which include treatment of HER2 positive (HER2+) metastatic breast cancer, early breast cancer (EBC), and metastatic gastric cancer [3-5]. Development and approval were based on a totality of evidence (TOE) approach, involving stepwise generation of comparative analytical (structural and functional), preclinical, and clinical (pharmacokinetics [PK], pharmacodynamics [PD], efficacy, safety and immunogenicity) evidence [6].
Scientific evidence in development of trastuzumab biosimilar ABP 980
Biosimilars/Research
|
Posted 15/05/2020
 0
Post your comment
0
Post your comment

Analytical characterization, the foundation of all biosimilar programmes, established structural and functional similarity of ABP 980 and trastuzumab RP [7, 8]. PK equivalence from a randomized, single-blind, single-dose, 3‑arm study that compared ABP 980 to trastuzumab RP in healthy subjects (n = 157) demonstrated geometric mean ratios and 90% confidence intervals (CIs) of key PK parameters, maximum serum concentration (Cmax) and area under the plasma concentration-time curve from time 0 to infinity (AUCinf), were contained within a prespecified bioequivalence margin of 0.80 to 1.25 (ABP 980 vs RP-US: 1.04 [0.99–1.08] and 1.06 [1.00–1.12]; ABP 980 vs RP-EU: 0.99 [0.95–1.03] and 1.00 [0.95–1.06]) [9]. Finally, a randomized, multicentre, double-blind, active-controlled clinical trial (LILAC) in women (n = 725) with HER2+ EBC established clinical similarity between ABP 980 and trastuzumab RP [10].
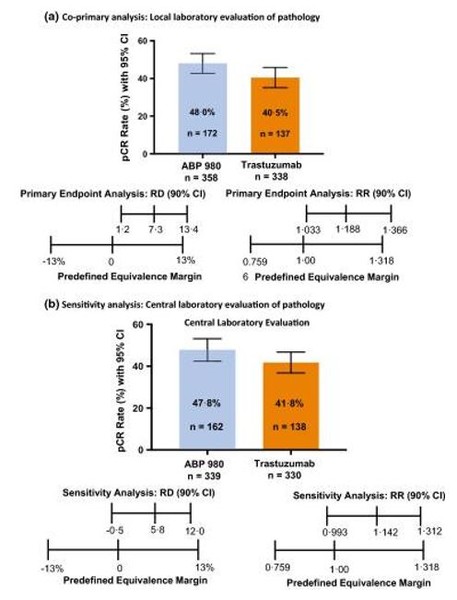
In the primary analysis, based on local pathology review, a higher proportion of subjects in the ABP 980 group achieved pathologic complete response (pCR) versus the RP (48% vs 41%; risk difference [RD]: 7.3%, 90% CI [1.2%−13.4%]; risk ratio [RR]: 1.188, 90% CI 1.033%−1.366%), allowing a conclusion of non-inferiority, see Figure 1a; however, non-superiority could not be confirmed. Nevertheless, sensitivity analyses from central laboratory review confirmed clinical similarity of ABP 980 and the RP (pCR: 48% vs 42%; RD: 5.8%, 90% CI –0.5%-12.0%; RR: 1.142, 90% CI 0.993%–1.312%), see Figure 1b. The study, conducted in both neoadjuvant and adjuvant settings, incorporated a single transition in the adjuvant phase, and demonstrated that switching from the RP to ABP 980 was safe, with similar incidences of adverse events, including cardiac symptoms. No new or unexpected safety signals emerged during neoadjuvant, adjuvant or switch phases, see Table 1, including similar declines in left-ventricular ejection fraction (2.8%, 3.3%, and 3.5% in the ABP 980, RP, and switch groups, respectively) [10, 11]. Immunogenicity, monitored by incidence of anti-drug antibodies, was also similar among patients in the ABP 980 (n = 2), RP (n = 2), and switch (n = 4) groups.
Figure 1: Comparative clinical study results of ABP 980 vs trastuzumab RP in HER2+ early breast cancer: Local (a) and central (b) laboratory evaluation of Total pCR [10]
CI = confidence interval; pCR = pathologic complete response; RD = risk difference; RP = reference product; RR = risk ratio.
Reproduced with permission from von Minckwitz et al, 2018 [10]. [permission received]
These results demonstrated that there were no clinically meaningful differences between ABP 980 and trastuzumab RP in efficacy, safety and immunogenicity. Overall, the TOE supported scientific justification for extrapolation of indications to all approved indications of the RP [6].
| Table 1: Comparative clinical study (LILAC trial) results of ABP 980 vs trastuzumab RP in HER2+EBC: all grade treatment-emergent adverse events [6] | |||||
| Neoadjuvant Phase | Adjuvant Phase | ||||
|
ABP 980 (n = 364) |
Trastuzumab RP (n = 361) |
ABP 980 (n = 349) |
Trastuzumab RP (n = 171) |
Switched from adjuvant trastuzumab RP to ABP 980 (n = 171) |
|
| Neutropenia | 53 (14.6%) | 45 (12.5%) | 25 (7.2%) | 10 (5.8%) | 6 (3.5%) |
| Arthralgia | 63 (17.3%) | 55 (15.2%) | 20 (5.7%) | 9 (5.3%) | 9 (5.3%) |
| Asthenia | 54 (14.8%) | 59 (16.3%) | 18 (5.2%) | 7 (4.1%) | 10 (5.8%) |
| Anaemia | 40 (11.0%) | 38 (10.5%) | 17 (4.9%) | 7 (4.1%) | 10 (5.8%) |
| Neuropathy peripheral | 51 (14.0%) | 43 (11.9%) | 8 (2.3%) | 3 (1.8%) | 2 (1.2%) |
| Adverse events of interest | |||||
| Infusion reactions | 80 (22.0%) | 68 (18.8%) | 28 (8.0%) | 14 (8.2%) | 20 (11.7%) |
| Neutropenia | 69 (19.0%) | 57 (15.8%) | 38 (10.9%) | 16 (9.4%) | 13 (7.6%) |
|
Infections and infestations |
51 (14.0%) | 55 (15.2%) | 54 (15.5%) | 17 (9.9%) | 23 (13.5%) |
| Hypersensitivity | 24 (6.6%) | 19 (5.3%) | 11 (3.2%) | 7 (4.1%) | 8 (4.7%) |
| Cardiac failure | 6 (1.6%) | 1 (0.3%) | 2 (0.6%) | 1 (0.6%) | 1 (0.6%) |
| Pulmonary toxicity | 1 (0.3%) | 1 (0.3%) | 4 (1.1%) | 2 (1.2%) | 1 (0.6%) |
| EBC: early breast cancer; HER2+: human epidermal growth factor receptor 2 positive; RP: reference product. | |||||
Conflict of interest
Several of the authors of the research paper [6] reported conflict of interest, including having received honoraria from, being a consultant for, or being an employee of pharmaceutical companies. For full details of the authors’ conflict of interest, see the research paper [6].
Abstracted by Hans-Christian Kolberg, MD, PhD, Marienhospital Bottrop gGmbH, Bottrop, Germany; and Dr Vladimir Hanes, Amgen Inc, USA.
References
1. GaBI Online - Generics and Biosimilars Initiative. US guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 May 15]. Available from: www.gabionline.net/Guidelines/US-guidelines-for-biosimilars
2. GaBI Online - Generics and Biosimilars Initiative. EU guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 May 15]. Available from: www.gabionline.net/Guidelines/EU-guidelines-for-biosimilars
3. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 May 15]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-the-US
4. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 May 15]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
5. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Japan [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 May 15]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Japan
6. Kolberg HC, Colleoni M, Santi P, et al. Totality of scientifc evidence in the development of ABP 980, a biosimilar to trastuzumab. Target Oncol. 2019;14(6):647‐56.
7. Hutterer K, McBride H, Polozova A, et al. Assessing analytical and functional similarity of proposed Amgen Biosimilar ABP 980 to trastuzumab. BioDrugs. 2019;33(3):321-33.
8. Jassem S, Wang W, Sweet H, et al. Functional and non-clinical similarity of ABP 980, a biosimilar of trastuzumab. Pharm Res. 2019;36(12):177.
9. Hanes V, Chow V, Zhang N, et al. A randomized single-blind, single-dose study evaluating the pharmacokinetic equivalence of proposed biosimilar ABP 980 and trastuzumab in healthy male subjects. Cancer Chemother Pharmacol. 2017;79(5):881-8.
10. von Minckwitz G, Colleoni M, Kolberg HC, et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19(7):987-98.
11. Kolberg HC, Colleoni M, Demetriou GS, et al. Cardiac safety of the trastuzumab biosimilar ABP 980 in women with HER2-positive early breast cancer in the randomized, double-blind, active-controlled LILAC study. Drug Saf. 2020;43(3):233-42.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2020 Pro Pharma Communications International. All Rights Reserved.
News
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment