Plavix (clopidogrel) has been a blockbuster drug. The lower cost of generic clopidogrel, available for the first time since 17 May 2012 [1], is intensifying debate among cardiologists over how to make sure patients get optimal benefit from any blood-thinning treatment.
Which antiplatelet agent? The debate intensifies
Generics/General
|
Posted 08/06/2012
 0
Post your comment
0
Post your comment

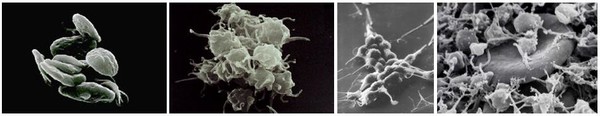
The debate hinges on the efficacy, as well as the cost, of the drug. In heart patients, normal platelets may become activated inside a blood vessel or stent, leading to clots that can obstruct blood flow, see Figure 1. The drug specifically and irreversibly inhibits the P2Y12 subtype of the adenosine diphosphate platelet collagen receptor, which is important in activation of platelets and eventual cross-linking by the protein fibrin.
Figure 1: Activated platelets trap an erythrocyte to form a blood clot.
Source: University of Oklahoma, Health Sciences Centre
The incidence of stent thrombosis is decreasing steadily. However, clopidogrel is a prodrug which has to be metabolised before it can work to prevent heart attack or stroke. It is thought that nearly a third of patients prescribed clopidogrel have a genetic variation that limits their response. So for these patients, some doctors prescribe prasugrel (Effient) or ticagrelor (trade name Brilinta in the US, Brilique and Possia in the EU) two rival drugs used by far fewer patients. Tests are available to assess a patient’s responsiveness to Plavix. But factors besides genetics, such as diabetes, can affect the drug’s performance.
A 423-patient German study published online in April 2012 by the Journal of the American College of Cardiology found that using a test to switch poor Plavix responders to Effient did not affect the number of heart attacks. To date, no studies have proved that identifying poor responders and switching them to alternative therapies reduces their risk. But the study enrolled stable patients undergoing a stent procedure and the results may not apply to higher-risk patients.
Some of the drug’s success is partly as a result of the combined treatment of patients with clopidogrel and aspirin. However, clopidogrel also interacts with a wide range of drugs, for example, proton pump inhibitors, which are routinely given with aspirin to prevent gastric ulcers and haemorrhages.
So the best treatment strategy is not easy to devise. Some researchers are looking into whether increasing the dose of clopidogrel can overcome the lack of response. This becomes more cost-effective as the cost of clopidogrel decreases.
In the absence of more definitive evidence, there will be pressure to steer patients away from more expensive strategies in favour of generic clopidogrel. ‘We have switching programs to help get patients to the lowest cost agent,’ says Dr Steve Miller, Chief Medical Officer of Express Scripts Inc, which manages prescriptions for 90 million Americans.
For those patients directly aware of the cost of the drug, it is hoped that a new lower price will significantly help compliance, the willingness to continue taking the drug over time.
Editor’s comment
Readers interested to learn more of the EU country experiences and perspectives of generic clopidogrel usage are invited to visit www.gabi-journal.net to view the following two peer reviewed articles due to be published in 2012, Issue 2, in GaBI Journal:
What lessons can be learned from the launch of generic clopidogrel?
Generic clopidogrel – the medicines agency’s perspective
If you are interested in contributing a research article in a similar area to GaBI Journal, please send us your submission here.
Related articles
2012’s biggest patent expiries
Apotex loses fight over Plavix patents
Reference
1. GaBI Online - Generics and Biosimilars Initiative. Clopidogrel goes off-patent: last of the old-style blockbusters [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Jun 8]. Available from: www.gabionline.net/Generics/General/Clopidogrel-goes-off-patent-last-of-the-old-style-blockbusters
Source: The Wall Street Journal
Research
Japan’s drug shortage crisis: challenges and policy solutions
Saudi FDA drug approvals and GMP inspections: trend analysis
EMA launches European shortages monitoring platform to tackle persistent medicine shortages

Generics/General Posted 03/12/2024
FDA releases one-year progress report for the Generic Drug Cluster

Generics/General Posted 28/10/2022
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment